Introduction
Hookworms are parasitic nematodes belonging to the family Ancylostomatidae, which includes two main genera: Necator americanus and Ancylostoma duodenale1. The hookworm life cycle consists of five larval stages (L1 to L5), beginning with the unembryonated eggs being shed in the soil. There, the eggs become fertile and eventually hatch into larvae, undergoing several developmental stages until they become infective adults. One or two days after the eggs embryonate, they release rhabditiform larvae, which are not yet infective. These larvae undergo two molts (L1 and L2) before transforming into infective filariform larvae (L3), which can survive in favorable environmental conditions for three to four weeks. Infection occurs when human skin, usually on bare feet, hands, buttocks, or back, comes into contact with soil contaminated by the infective larvae. The larvae penetrate the skin and enter the bloodstream through the capillaries. From there, they are passively transported to the right chambers of the heart and then to the pulmonary vasculature, where they cross into the alveoli. Once in the lungs, the L3 larvae exit the alveoli and migrate up the bronchial tree, ascending to the pharynx, where they are swallowed. They then enter the gastrointestinal tract, eventually reaching the small intestine, where they complete their migration. Once in the small intestine, the larvae undergo two additional molts (L4 and L5) and use their teeth or cutting plates to lacerate the mucosa and anchor themselves, facilitating feeding while preventing expulsion by intestinal peristalsis. As they begin to feed on blood, the juvenile parasites mature into adult hookworms (L5), which can live for many years in the small intestine of their human hosts1,2.
Morphologically, N. americanus has a pair of cutting plates in its buccal capsule, while A. duodenale has a pair of fangs that it uses to attach to the mucosa of the small intestine for feeding. Mucosal injury causes inflammation and bleeding, and the resulting blood loss can lead to iron deficiency anemia in individuals hosting moderate to high numbers of adult parasites1. The adult parasite measures between 5 and 13 mm, and both species cause bleeding. However, it has been shown that A. duodenale is responsible for a blood loss 10 times greater than that caused by N. americanus2. The greatest blood loss occurs around the site where the parasite attaches to the host’s mucosa. The parasite secretes anticoagulant substances that inhibit factors XIa, VIIa, and tissue factor, disrupting hemostasis at the site of injury3. It is estimated that N. americanus can cause a blood loss of 0.03 to 0.04 mL/day, and 25 adult parasites can lead to a blood loss of 1 mL/day, while A. duodenale can cause a blood loss of 0.15 to 0.20 mL/day, with just 5 adult parasites causing a loss of 1 mL/day of blood4.
The prevalence of hookworm infection is influenced by environmental, socioeconomic, and personal factors5. It is estimated that approximately 500 million people worldwide, living in tropical regions, are infected with hookworms, causing more than 4 million disability-adjusted life years (DALYs). Additionally, hookworm infection represents an economic burden, with estimated losses exceeding USD 130 billion annually1,6. In Colombia, the prevalence of hookworm infection among children has been estimated at 6.4%. In the biogeographical province of Chocó-Magdalena, which includes the department of Cauca, the prevalence of hookworms was estimated at 9.8%, second only to the department of Amazonas, which had the highest prevalence at 35.7%7. In the indigenous population of the department of Cesar, a prevalence of 18% for N. americanus was reported, while in Córdoba, 22.5% of the subjects studied were found to be infected with hookworms8,9.
Symptoms associated with hookworm infection can include cutaneous manifestations, pulmonary symptoms (Loeffler’s syndrome due to the L3 larval stage), abdominal pain, nausea, iron deficiency anemia, malnutrition, and anasarca due to plasma hypoproteinemia5. Hookworm disease is treatable, and recovery is complete. Diagnosis is traditionally made by detecting eggs in stool samples or through molecular methods1. Below, we present a case of intestinal bleeding and severe anemia caused by hookworms, diagnosed via capsule endoscopy in Colombia.
Clinical Case
This is the case of a 38-year-old male patient from Boca Grande, López de Micay, Cauca, Colombia, with a history of weekend binge drinking, smoking, and having undergone a thoracotomy and laparotomy five years ago due to a gunshot wound. He sought care at a Level I health center after experiencing a one-month history of gastrointestinal bleeding, characterized by melena with occasional rectal bleeding, along with asthenia, adynamia, objective vertigo, blurred vision, precordial pain, and shortness of breath with mild exertion, which worsened in the last three days. Upon initial evaluation, his hemoglobin (Hb) was found to be 2.8 g/dL, and based on his symptoms, the working diagnosis was gastrointestinal hemorrhage. He was treated with intravenous fluids and tranexamic acid and was urgently referred to a higher-level care facility for further management.
Upon admission to the Level III health center, the patient was in fair general condition. He denied abdominal pain, chest pain, or difficulty breathing. On physical examination, his skin was pale, and his oral and conjunctival mucosa were pale and dry. A digital rectal exam revealed black material on the glove, suggestive of melena, without pain or masses. His vital signs were as follows: blood pressure: 80/40 mm Hg, heart rate: 90 beats per minute (bpm), respiratory rate: 16 breaths per minute, temperature: 36.0 °C, ambient oxygen saturation: 90%, glucose: 109 mg/dL, Glasgow Coma Scale: 15/15. The patient weighed 69 kg and was 1.60 meters tall. Laboratory results showed the following: hemoglobin (Hb): 2.5 g/dL (normal range: 13.6-17.5), hematocrit: 8.9% (39.5-50.3), reticulocytes: 2.43%, white blood cells: 3,700/µL (4,000-11,200), neutrophils: 2,600/µL (1,800-6,400), lymphocytes: 600/µL (1,300-3,500), eosinophils: 100/µL (100-500), mean corpuscular volume (MCV): 52.1 fL (80.7-95.5), mean corpuscular hemoglobin concentration (MCHC): 27.9 g/dL (32.5-35.2), platelets: 391,000/µL (159,000-388,000), prothrombin time (PT): 15.2 seconds, partial thromboplastin time (PTT): 33.6 seconds, international normalized ratio (INR): 1.36, and albumin: 3.51 g/dL. Electrolytes, renal function, and liver function were within normal limits. Given the patient’s hemodynamic state and low hemoglobin level, treatment for hypotension and gastrointestinal bleeding was initiated. This included 1000 mL of Hartmann’s solution followed by an infusion at 80 mL/h, intravenous omeprazole 80 mg followed by 40 mg IV every 12 hours, 1 g IV tranexamic acid, 10% calcium gluconate IV every eight hours, and due to severe anemia, three units of emergency packed red blood cells were transfused.
The patient was evaluated by the general surgery team, and it was initially considered to perform an upper gastrointestinal endoscopy (UGIE). The exploration, up to the second portion of the duodenum, revealed chronic antral gastritis and pale gastric mucosa, without evidence of recent or old bleeding. Given the negative results from the first UGIE, a total colonoscopy was considered to explore the lower digestive tract. The colonoscopy findings included nonspecific colitis, possibly of infectious origin, and grade I hemorrhoids, with no evidence of bleeding lesions. Treatment was initiated with metronidazole 500 mg IV every eight hours, ciprofloxacin 500 mg orally (PO) every 12 hours, and mesalazine 500 mg PO every eight hours. During the hospital stay (10 days) and until the definitive diagnosis of the cause of the bleeding was made, the patient received three transfusions of packed red blood cells (Table 1). Following the institutional protocol, a second UGIE was performed, which was negative for bleeding lesions up to the second portion of the duodenum. Additionally, stool tests and Wright’s stain were negative for the presence of eggs and blood parasites, respectively. Since the cause of the intestinal bleeding remained unidentified, exploration of the small intestine via capsule endoscopy was considered.
Table 1 Hemoglobin levels and results of endoscopic studies performed on the patient with severe anemia syndrome
| Date | Transfused Blood Products | Hb Level | Blood Pressure (mm Hg) | Digestive Exploration |
|---|---|---|---|---|
| 12/14/2022 | Three PRBC units | 2.5 g/dL (at admission) | 80/40 | First UGIE: chronic antral and corporal gastritis, negative for bleeding |
| 12/15/2022 | 5.0 g/dL | 110/75 | ||
| 12/16/2022 AM | Two PRBC units Four FFP units | 6.7 g/dL | 116/73 | Total colonoscopy: colitis of possible infectious origin, grade I hemorrhoids, negative for lower gastrointestinal bleeding |
| 12/16/2022 PM | 1 PRBC unit | 6.9 g/dL | 118/75 | |
| 12/17/2022 | 8.0 g/dL | 114/78 | Second UGIE: chronic antral and corporal gastritis, negative for bleeding | |
| 12/19/2022 | 8.1 g/dL | 120/88 | ||
| 12/20/2022 | 9.2 g/dL | 136/89 | Capsule endoscopy: severe nematode infestation of the small intestine suggestive of hookworms, no signs of bleeding or other lesions | |
| 12/21/2022 | 8.8 g/dL | 118/78 | ||
| 12/22/2022 | 8.9 g/dL | 115/68 | ||
| 12/23/2022 | 8.9 g/dL (discharge) | 123/78 |
UGIE: upper gastrointestinal endoscopy; PRBC: packed red blood cells; FFP: fresh frozen plasma. Author’s own research.
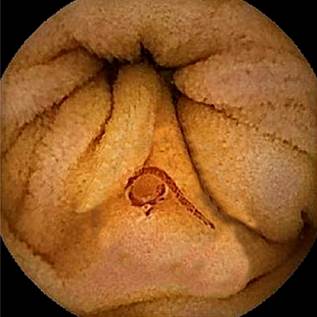
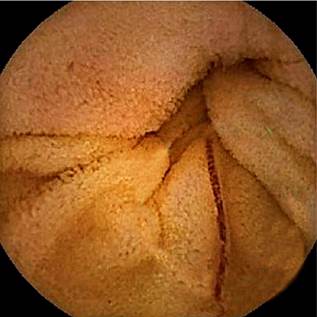
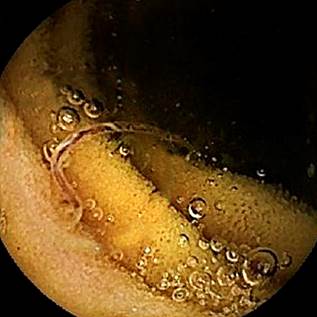
After bowel preparation with a laxative, a capsule endoscopy was performed, revealing the presence of a significant number of cylindrical worms in the small intestine, mostly located in the proximal jejunum. No parasites were identified in the duodenum. The worms were either dark red or light red in color, and some were anchored to the intestinal wall (Figures 1-4). The rest of the examination was normal, with no active bleeding lesions documented in the intestinal mucosa. Based on the macroscopic morphological characteristics of the parasites, their location, and the patient’s symptoms, the parasites were identified as hookworms. With this finding, and given the patient’s favorable clinical evolution, including hemodynamic stability, stable hemoglobin levels, the absence of new episodes of gastrointestinal bleeding, and no further need for blood transfusions, the patient was discharged with outpatient management. He was prescribed albendazole 400 mg/day for five consecutive days, with a recommendation to repeat the dose after 21 days. Additionally, esomeprazole 40 mg/day was prescribed for 60 days, and follow-up was arranged via outpatient service.
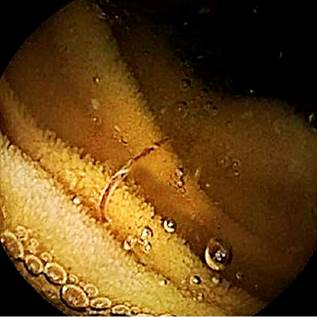
Author’s File.
Figure 3 Another image from the capsule endoscopy showing a hookworm in the patient’s small intestine.
Discussion
Hookworms were first described and classified in 1838 by Italian physician Angelo Dubini, who discovered these parasites in the small intestine during an autopsy10. Since then, hookworms have been a growing focus of interest due to their negative impact on human health. This parasite, also referred to as ancylostomiasis, belong to the nematode family Ancylostomatidae, with the most representative species being A. duodenale, N. americanus, Ancylostoma caninum, and Ancylostoma braziliense. All of these species can cause disease in humans; however, A. caninum and A. braziliense also affect dogs and cats, respectively, and represent zoonotic infections in humans, where filariform larvae penetrate the skin and cause cutaneous larva migrans syndrome1.
The primary hookworms that affect humans are A. duodenale and N. americanus. Transmission occurs through skin contact with contaminated soil, and environmental and socioeconomic factors, such as improper fecal waste management, humid environments, warm temperatures, and outdoor activities performed barefoot, facilitate the transmission of these parasites1. Additionally, socioeconomic conditions such as poverty, poor water quality, and inadequate sanitation contribute to the transmission and prevalence of this geohelminthiasis, which is widespread and most common in tropical and subtropical regions2,11.
Once the filariform larvae penetrate the skin, they migrate, and the adult parasites settle in the host’s small intestine, where they damage the intestinal mucosa. As a defense mechanism, the human body mounts a robust immune response against the parasites, driven by Th2 cells and immunoglobulin E (IgE) antibodies. However, despite this immune response, a significant number of parasites survive and persist in the human host for years1,12. In the intestinal mucosa, hookworms feed on the host’s blood, leading to iron deficiency anemia. Experimentally, hookworms’ ability to cause anemia has been demonstrated by infecting 10 healthy subjects with a gelatin capsule containing 150 A. duodenale larvae in the L3 stage. A decrease in hemoglobin (Hb) levels was observed, dropping from an average pre-infection Hb of 13 g/dL (range: 12-15 g/dL) to a post-infection average of 10.4 g/dL (range: 9.8-11 g/dL) over three months10,13. Additionally, the severity of anemia has been shown to depend on the type of infecting parasite, with infection by A. duodenale resulting in greater blood loss than that caused by N. americanus5,14.
On the one hand, most individuals infected with hookworms present with chronic, asymptomatic occult gastrointestinal bleeding; however, in rare cases, they can cause acute anemia15,16. Hookworm infections are often overlooked or misdiagnosed, making it essential to consider these parasites in the differential diagnosis of anemia and acute or chronic gastrointestinal bleeding, particularly in patients with risk factors who come from rural areas of tropical developing countries1,17,18. Additionally, hookworms can also lead to hypoalbuminemia, elevated IgE levels, and eosinophilia1. In the present case, eosinophilia and hypoalbuminemia were not identified, but the patient did present with microcytic hypochromic anemia. Eosinophilia in hookworm infection is typically detected within the first 20 to 30 days post-infection, peaking between 6 and 12 weeks, and then gradually returning to normal levels1,13). The absence of eosinophilia in this case may suggest a chronic hookworm infection.
On the other hand, upper gastrointestinal bleeding, which includes lesions from the upper esophageal sphincter to the ligament of Treitz, is considered the most prevalent gastroenterological emergency. The incidence of upper gastrointestinal bleeding in the United States has been estimated between 50 and 172 cases per 100,000 people per year; in Spain, 34 cases per 100,000; in the United Kingdom, 72 cases per 100,000; and in Malaysia, 100 cases per 100,000. Mortality rates due to upper gastrointestinal bleeding have been estimated at 4% to 14% in China, 9% in Peru, 4% in Mexico, and between 5% and 10% in Colombia19. Similarly, the overall incidence of lower gastrointestinal bleeding is estimated between 33 and 87 cases per 100,000 people20, while small intestine bleeding accounts for 5% to 10%21. Both upper and lower gastrointestinal bleeding can be investigated using upper gastrointestinal endoscopy (UGIE) or colonoscopy. However, exploring the small intestine has historically been challenging21. Fortunately, capsule endoscopy has enabled the exploration of the small intestine in cases of suspected obscure gastrointestinal bleeding, with a diagnostic yield of 50% to 60% and a negative predictive value of 90%21,22.
In the present case, the patient presented with obscure gastrointestinal bleeding, suggesting a source from the upper or middle intestine. However, UGIE ruled out bleeding lesions in the upper intestine, and the colonoscopy did not reveal any active bleeding lesions in the colon but did report the presence of grade I hemorrhoids, which could explain the episodes of rectal bleeding reported by the patient.
On the other hand, stool examination is the most commonly used method for diagnosing hookworms; however, the sensitivity of this method using Kato-Katz and formalin-ether concentration techniques is 68.4% (95% confidence interval [CI]: 56.6-78.3) and 38.2% (95% CI: 27.5-50.1), respectively, indicating low sensitivity23. In Colombia, the sensitivity of the Kato-Katz method for hookworms has been estimated at 73%24. Several case reports describe the diagnosis of intestinal hookworms via capsule endoscopy; however, to date, no such reports have been published in Colombia (Table 2). In two of these cases, stool tests were positive for eggs, and in two other cases, no stool tests were mentioned. However, in all cases, capsule endoscopy demonstrated the presence of hookworms in the intestine, and in four cases, the stool test results were negative for hookworm eggs, as was the case in the present report. These data suggest that the sensitivity of stool tests for hookworms does not exceed 80% and indicate that capsule endoscopy may provide a complementary method for diagnosing hookworm infections15,24,25.
Table 2 Clinical characteristics and test results of patients with hookworms diagnosed via capsule endoscopy
| Author | Clinical Characteristics | UGIE | Colonoscopy | Capsule Endoscopy Findings | Presence of Hookworm Eggs in Stool |
|---|---|---|---|---|---|
| Wu, 200726 | 39-year-old man with watery diarrhea | Not performed | Hookworms in the ascending colon | Hookworms in the stomach, duodenum, and jejunum | Positive |
| Kalli, 201127 | 22-year-old man with severe iron deficiency anemia | Normal | Normal | Hookworms in the proximal small intestine, slight bleeding at parasite attachment site | Negative |
| Seidelman, 201525 | 63-year-old man with generalized weakness, loss of appetite, iron deficiency anemia | Mild non-erosive gastritis | Internal hemorrhoids grade I, melena | Hookworms in the mid-jejunum | NR |
| Coton, 201728 | 43-year-old man with unexplained iron deficiency anemia | Normal | Normal | Duodenal bleeding, linear jejunal ulcer, hookworms | Negative |
| Tan, 201715 | 46-year-old man with acute hematochezia | Hookworms in the duodenum | Normal | Fresh bleeding in the jejunum, hookworms in the jejunum | NR |
| Meng, 201829 | 46-year-old man with acute hematochezia | Hookworms in the duodenum | Normal | Hookworms in the jejunum, active bleeding, blood clots | Negative |
| Chergui, 202130 | 34-year-old man with severe iron deficiency anemia | Normal | Normal | Hookworms in the proximal small intestine | Positive |
| Li, 202317 | 72-year-old man with chronic anemia and black stools | Chronic superficial gastritis, hiatal hernia | Internal hemorrhoids | Eroded jejunal mucosa with minimal bleeding, hookworms in the jejunum | Negative** |
| Present case | 38-year-old man with severe iron deficiency anemia | Chronic antral gastritis | Grade I hemorrhoids, nonspecific colitis | Hookworms in the jejunum | Negative |
*The patient underwent several stool tests, all of which were negative. **Hookworm eggs detected using FA160 automatic analyzer. NR: not reported. Adapted from: Tan X, and colleagues. 201715; Li B, and colleagues. 202317; Seidelman J, and colleagues. 201625; Wu IC, and colleagues. 200726; Kalli T, and colleagues. 201127; Coton T, and colleagues. 201828; Meng Y, and colleagues. 201829; Chergui H, and colleagues. 202130.
Hookworm disease is treatable, and recovery is complete. The two most widely used medications are albendazole and mebendazole, both of which are benzimidazoles. These drugs are broad-spectrum anthelmintics that work by inhibiting microtubule polymerization through binding to β-tubulin in invertebrates, effectively killing adult worms1. A single dose of 400 mg albendazole is more effective than a single 500 mg dose of mebendazole. A systematic review and meta-analysis reported that a single dose of albendazole has a cure rate of 72% (95% CI: 59-81), while a single dose of mebendazole has a cure rate of only 15% (95% CI: 1-27)31. Similarly, a study involving 1,834 school-aged children from several countries showed an overall cure rate for hookworm infection of 87.8% with a single dose of albendazole32. Additionally, three consecutive daily doses of albendazole achieved a 92% cure rate (95% CI: 80.8-97.8), compared to 58.5% (95% CI: 45.6-70.6) for mebendazole33. In this case, the decision was made to prescribe five consecutive days of treatment with albendazole and to repeat a 400 mg dose at 21 days to improve the cure rate.
Conclusion
In patients from rural areas with a high prevalence of geohelminthiasis who present with acute or chronic gastrointestinal bleeding and show evidence of iron deficiency anemia, hookworm infection should be considered in the differential diagnosis. Capsule endoscopy can help identify the cause of gastrointestinal bleeding and rule out intestinal parasitic disease.











 text in
text in