Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Agronomía Colombiana
Print version ISSN 0120-9965
Agron. colomb. vol.34 no.2 Bogotá May/Aug. 2016
https://doi.org/10.15446/agron.colomb.v34n2.55279
Doi: http://dx.doi.org/10.15446/agron.colomb.v34n2.55279
Growth and phenology of three Andean potato varieties (Solanum tuberosum L.) under water stress
Crecimiento y fenología de tres variedades andinas de papa (Solanum tuberosum L.) en estrés hídrico
Loyla Rodríguez P1, Danny Sanjuanelo C.2, Carlos Eduardo Ñústez L.3, and Liz Patricia Moreno-Fonseca3
1 Departamento de Biología, Facultad de Ciencias, Pontificia Universidad Javeriana. Bogotá (Colombia). loyla.rodriguez@javeriana.edu.co
2 Faculty of Sciences, Universidad de Ciencias Aplicadas y Ambientales (UDCA). Bogotá (Colombia).
3 Department of Agronomy, Faculty of Agricultural Sciences, Universidad Nacional de Colombia. Bogotá (Colombia).
Received for publication: 21 January, 2016. Accepted for publication: 30 June, 2016.
ABSTRACT
The water-deficit stress has a negative effect on the growth and development of plants, reducing the yield of crops. This study evaluated the effect of a water deficit on the growth and phenology of potato (Solanum tuberosum L.) varieties Diacol Capiro, Pastusa Suprema and Esmeralda. Plants that were starting tuberization were subjected to a water deficit by suspension of irrigation until reaching a foliar water potential of -2.0 MPa; later the plants were re-irrigated and recovered. The water deficit decreased the flowering time in 'Diacol Capiro', the development of leaves and maturation of fruits in 'Esmeralda' and the development of leaves and formation of lateral shoots in 'Pastusa Suprema'. In the three varieties, the water deficit did not induce a significant reduction in the stem length, the number of leaves per stem and per site or the number of main stems per site. The plants demonstrated responses related to escape and evasion mechanisms during the water deficit through the adjustment of the metabolism in order to reduce the duration of the phenological stages. The duration of the biological cycle for the three varieties was 148 days, with a requirement of 1,850 GDD. There were no differences in the potential yield, probably due to the short duration of the stress period. The three varieties demonstrated plasticity when modifying the phenology in response to the drought period.
Key words: Drought, plant development, stress escape, growing degree-day.
RESUMEN
La sequía en el suelo es un factor ambiental que modifica la fenología, el crecimiento y la productividad de los cultivos. Este estudio evaluó el efecto del déficit hídrico sobre el crecimiento y desarrollo de variedades de papa (Solanum tuberosum L.) Diacol Capiro, Pastusa Suprema y Esmeralda. Las plantas en la inducción de la tuberización, se sometieron a estrés hídrico por suspensión del riego hasta alcanzar un potencial hídrico foliar de -2,0 MPa; las plantas se regaron de nuevo y se recuperaron. El déficit hídrico redujo el tiempo de floración de 'Diacol Capiro', el desarrollo de hojas y la maduración del fruto en 'Esmeralda' y el desarrollo de hojas y formación de brotes laterales en 'Pastusa Suprema'. En las tres variedades, el déficit hídrico no indujo una reducción significativa en la longitud del tallo, el número de hojas por tallo y por sitio o el número de tallos principales por sitio. Las variedades mostraron respuestas relacionadas con el mecanismo de escape o evitación del periodo de déficit hídrico mediante la acomodación del metabolismo para acortar la duración de los estadios fenológicos. La duración del ciclo biológico para las tres variedades fue de 148 días, con un requerimiento de 1,850 GDD. No hubo diferencias en el rendimiento potencial, probablemente debido a la corta duración del período de estrés. Las tres variedades demostraron plasticidad al modificar la fenología en respuesta al periodo de sequía.
Palabras clave: Sequía, estados de desarrollo, escape, grados calor.
Introduction
Increases in global temperatures and decreases in precipitation are having a negative effect on the availability of water resources for the planet (Oki and Kanae, 2006). This limitation causes droughts, affecting the growth and development of plants and causing a decrease in the productivity of crops worldwide (Körner and Basler, 2010; Hossain et al., 2012).
Reductions in growth have been reported in numerous plants under water deficit conditions, including for economically important crops, such as the potato (Lahlou et al., 2003; Yuan et al., 2003; Ierna and Mauromicale, 2006), sorghum (Zegada-Lizarazu and Monti, 2013) and rice (Lilley and Fukai, 1994). Likewise, it has been found that water deficits modify the phenology of plants, such as soy (Desclaux and Roumet, 1996), rice (Lilley and Fukai, 1994) and raspberry (Morales et al., 2013). It is known that plants respond to water deficit stress through different escape and tolerance mechanisms (McDowell et al., 2008). One strategy involves reducing the duration of the life cycle (Wang et al, 2006; McDowell et al., 2008; Touchette et al., 2009). In potato, water deficits reduce the growth period of the foliage through early maturation of the plant, causing a reduction in the life cycle and yield (Darwish et al., 2006; Kuppinger et al., 2014). A determining factor for the growth and development processes of plants is temperature and its effect has been studied in plants, such as bean (Barrios-Gómez and López-Castañeda, 2009), melon (Bouzo and Küchen, 2012) and potato (Struik et al., 1989). Plants require a certain amount of heat to develop from one point in their life cycles to another; measuring the accumulated heat is known as physiological time and is often expressed in units called growing degree-days (GDD) (Bonhomme, 2000; Mazurczyk et al., 2003; Barrios-Gómez and López-Castañeda, 2009). Degree-days accumulate from the base temperature, which is the temperature below which plant growth is zero (Bonhomme, 2000; Timlin et al., 2006; Bouzo and Küchen, 2012); while, the optimal temperature is the temperature where the maximum rate of a process is reached (Yamasaki et al., 2002).
Thus, the effect of temperature on the plant development process could be studied measuring the heat necessary, in degree-days, to develop from one phenological state to another (Villaseca et al., 1986). Different studies have been carried out to determine the thermal time requirements for the phenological development of different potato genotypes (Sale, 1979; Ewing, 1981; Mazurczyk et al., 2003; Timlin et al., 2006). However, few Andean potato varieties have been characterized with respect to thermal time requirements for phenological development (Gutiérrez, 1985; Villaseca et al., 1988). There are only a few studies that have assessed the effect of water stress on plant phenology, which include studies on soy (Desclaux and Roumet, 1996), rice (Lilley and Fukai, 1994) and raspberry (Morales et al., 2013), but there are no reports on potato plants.
Solanum tuberosum L. is a widely cultivated species in the world, due to its dietary importance (Kuiper et al., 2001). In Colombia and other countries of South America, this species is cultivated in the upland areas of mountainous terrain, with little or no availability of irrigation, so it is often subjected to water deficit. Currently, there is no information about the effect of water deficits on the growth and phenology of potato varieties, knowledge that is necessary to implement management strategies for this crop under conditions of water deficit stress. The aim of this study was to determine the effect of water deficits at the beginning of tuberization on the phenology and growth degree-day requirements, parameters of growth and yield potential in the Colombian potato varieties (Solanum tuberosum L.) Diacol Capiro, Pastusa Suprema and Esmeralda.
Materials and methods
Plant material and experiment design
This study was carried out in 2013 in a greenhouse at the Faculty of Agricultural Sciences of the Universidad Nacional de Colombia, at 2,600 m a.s.l., with an average temperature of 17.43°C and a mean relative humidity of 76.24%. Tuber-seed potatoes of the varieties Diacol Capiro (CAP), Pastusa Suprema (SUP) and Esmeralda (ESM) were planted in black plastic bags with 5 kg of soil. Fertilization and agronomic management were carried out following commercial recommendations. During the experiment, the maximum and minimum temperatures and relative humidity (HR) were registered daily with a weather station (MCR200 μMetos®, Weiz, Austria).
The treatments were distributed in a split-plot arrangement under a complete block randomized design with three replications; the cultivars were placed in the main plots and the water state was placed in the subplots (water deficit or normal irrigation). In the water deficit treatment, irrigation was suspended at 74 d after sowing, during the start of the tuberization stage (King et al., 2004; Liu et al., 2005; Teixeira and Pereira, 2007). When the plants presented a foliar water potential (Ψh) close to -2.0 MPa (Van den Bilcke et al., 2013), the plants were re-irrigated and recovered.
The water deficit was applied for a short period of 4-6 d, until soil matric potential values near -45 kPa were reached, which are known to cause water-deficit stress in potato (Kawakami et al., 2006; Wang et al., 2007; Aksic et al., 2014). The stress level was also determined by measuring the foliar Ψh, for which values below -1.6 MPa have been reported, such as the permanent wilting point for potato plants (Vos and Haverkort 2007; Jensen et al., 2010; Rolando et al., 2015). The water deficit treatment was conducted until a foliar close to -2.0 MPa was reached (4 d for CAP, 5 for SUP and 6 d for ESM), after which the plants were re-irrigated.
Phenology and growing degree-day requirements
During the life cycle of each variety, changes in the vegetative and reproductive development were recorded in order to establish the main phenological stages, from sprouting to senescence, according to the BBCH scale (Meier, 2001).
The occurrence of a phenological stage was recorded when 50% of the plants reached the specific growth stage: sprouting, leaf development, formation of lateral shoots, stem elongation, tuber formation, inflorescence and flower development, fruit and seed development and senescence. During the life cycle of the crop, six plants per treatment were observed for the following: the day of sprouting, the number of leaves per plant with more than 4 cm2 of leaf area expansion, the number of secondary stems longer than 10 cm, the number and length of the main stems longer than 10 cm, the percentage of foliage coverage, the duration of the tuber bulking stage, the number of flower buds longer than 5 mm, the number of open floral buds per plant, the number of berries per plant, the shape and color of the berries and the stem and leaf coloration.
In addition, every 15 d, the following growth parameters were recorded: stem length from the root collar to the shoot apical meristem, number of leaves per stem and per sowing site (direct count), number of main stems per sowing site and, at 164 d, the potential yield was determined with the fresh weight of the tubers from 10 plants per treatment.
In each phenological stage, the growing degree-days (GDD) were calculated with Eq. 1, by Arnold (1959) and Juskiw et al. (2001):
where Tmax is the maximum daily air temperature, Tmin is the minimum daily air temperature and Tbase is the base temperature of 6°C, as reported for this crop (Cao and Tibbitts, 1995).
Statistical analysis
The evaluated variables were analyzed with an ANOVA and the means were compared with a Tukey test (P≤0.05). The data were processed with R language (R Development Core Team, 2010).
Results and discussion
During the experiment, the average minimum and maximum air temperature were 10.54±1.3°C and 34.51±3°C, respectively, and the mean minimum and maximum relative humidity were 46.97±10.2% and 98.81±1.56%, respectively. The mean vapor pressure deficit (VPD) was 0.38 (±0.8) KPa (Fig. 1).
Growing degree day requirements
The period between sprouting and end of flowering was shortened both by the water deficit and the maximum daytime temperature for the CAP and SUP varieties; while for the ESM, it was equal (Tab. 2; Fig. 2). Because the water deficit was reached by the varieties in a short period of time (4-6 d), when the flowering started, these varieties had been submitted to two days of water deficit, which probably induced shortening of the flowering. On the other hand, the maximum daytime temperature was above 30°C, which likely reduced the duration of inflorescence and flower development (Muthoni et al, 2012; Almekinders and Struik, 1994). Probably the most determinant factor was the interaction between the water deficit and the maximum temperature because the control plants did not present a shortening of the flowering. For CAP, it was 82 d for the irrigated plants (931 GDD) and 76 d (867 GDD) for the water deficit plants. For the irrigated SUP, this period was 76 d (867 GDD) and, for the plants in water deficit, it was 70 d (794 GDD); in the ESM, under both water regimens, it lasted 98 d (1118 GDD). On the other hand, the water deficit also accelerated flowering in the CAP and SUP varieties by 6 d. The growth cycle for the CAP, ESM and SUP, irrigated and under water deficit, was 148 d (1677 GDD) (Tab. 2; Fig. 2); lower data (165 d for ESM and CAP and 180 d for SUP) were found by Ñústez et al. (2011). This decrease was probably due to the conditions in the greenhouse: the average air temperature was between 17 and 22.98°C, exceeding the optimum temperature for the growth and development of S. tuberosum L. plants as reported by Sarquís et al. (1996) and Yuan and Bland (2004), between 15 and 18°C on the ground and in the air, and higher than that reported for the production areas of Cundinamarca, between 12 and 18°C (Fedepapa, 2004).
Sprouting. Sprouting was 50% for the three varieties at 13 days (136 GDD), with a mean temperature of 17.33°C (Tab. 2 and Fig. 2). Segura et al. (2006) reported 50% sprouting in CAP and SUP at 15 d and at 30 d for ESM. In this study, the sprouting occurred faster than as reported by Segura et al. (2006), possibly because the temperature during the growth stage (17.3°C) exceeded that reported as the optimal temperature (15 to 18°C) for the growth and development of S. tuberosum L. plants (Sarquís et al., 1996; Yuan and Bland, 2004).
Leaf development. The development of the leaves in the three varieties started at 13 d (136 GDD). For CAP under both water regimens, it had a duration of 85 d (1,116 GDD) and, for the irrigated ESM, it was 98 d (1,258 GDD), while in the water deficit ESM, it was 85 d (1,116 GDD) (Tab. 2; Fig 2). The development of leaves in SUP lasted 126 d (1,501 GDD) in the irrigated plants and 98 d (1,258 GDD) in the plants with a water deficit (Tab. 2, Fig. 2). Segura et al. (2006) reported a leaf development of 81 d for CAP, 109 d for SUP, and 95 d for ESM, differing from the present findings because the growth conditions were different in the two studies. The water deficit shortened the duration of the foliage growth, causing early maturation in the SUP and ESM (Van Loon 1981; Darwish et al, 2006). The average air temperature of 18±0.6°C for this growth stage was higher than that reported as optimal (between 15 and 18°C) for S. tuberosum L. (Sarquís et al., 1996; Yuan and Bland, 2004). The CAP variety leaf development was not affected by water deficit; probably the short period water deficit was not enough to shorten this stage of development.
Formation of lateral shoots. The three varieties began forming lateral shoots at 27 d (283 GDD) and the growth stage was not affected by the water deficit, and lasted 43 d (794 GDD) for CAP, 94 (931 GDD) for ESM and 49 d (867 GDD) for SUP (Tab. 2). This state of formation of lateral shoots started before the application of the water deficit (to the 74 das); therefore, the growth stage was probably not affected by the water deficit. Segura et al. (2006) reported the start of the formation of lateral shoots in the same varieties at 29 days after sprouting (das) for SUP and CAP and at 26 dasp for ESM, similar to the present study (Tab. 2, Fig. 2).
Main stem elongation. The stem growth began, in the three varieties, at 13 d (136 GDD) and this was not affected by the water deficit; the duration of the stem growth was 85 d (1,116 GDD) for CAP, 108 d (1,367 GDD) for ESM and 91 d (1,181 GDD) for SUP (Tab. 2; Fig. 2). The duration of this stage for the three varieties was higher than that reported by Segura et al. (2006): 59 d for CAP, 81 d for EMS and 67 d for SUP, under field conditions. The average daily temperatures of 19.33°C (CAP), 19.28°C (SUP) and 19.28°C (ESM) in the greenhouse, was higher than that reported for potato crops (Fig. 1), promoted the development of stems for a longer period. Stem elongation depends on diurnal temperature fluctuations (Erwin and Heins, 1995) and it has been reported that low intensity light induces stem elongation through increased cell elongation (Flores-López et al., 2009).
Tuber formation. The three varieties began tuberization at 70 d (794 GDD), which lasted 78 d (1677 GDD) and was equal in both the irrigated plants and the plants under water deficit (Tab. 2, Fig. 2). Segura et al. (2006) reported the start of tuberization at 35 dasp, lasting 102 d for CAP, 104 d for SUP and 96 d for ESM. The tuber bulking found in this study was lower at 24 d (CAP), 26 d (SUP) and 18 d (ESM) because tubers are inhibited at high temperatures (15 to 27°C) (Ewing, 1981; Struik et al, 1989). The average temperature was 19.16°C, higher than the average in the areas of production of these varieties, and generated a decrease in the duration of the tuber. This reduction, as well as the decrease in the quality and quantity of tubers and yield (less than 1 kg/plant), may have been due to the decrease in the assimilate partition resulting from the high temperature, which has been reported for long-day potato varieties (Ewing 1981) (Fig. 3). The GDD required for bulking the tubers in the evaluated short-day varieties (short-day) was higher (794-1,677 GDD), as described for long day S. tuberosum L. (Russet Burbank), which is between 2,000 and 2,220 GDD (Goeser et al, 2012).
Inflorescence and flower development. The emergence or appearance of flower buds began at 48 d for CAP, at 41 d for ESM and 48 d for SUP. Blooming, recorded with the presence of open flowers (Meier, 2001), in CAP lasted 12 d for the irrigated plants and 6 d for the plants under water deficit; for ESM, under both water regimens, it lasted 43 d; and for SUP, it was 21 d for the irrigated plants and 15 d for the plants in water deficit (Tab. 2, Fig. 2). Segura et al. (2006) reported a flowering duration for CAP of 56 d, 69 d for ESM and 41 d for SUP. The optimum temperature that promotes flowering induction in long-day varieties should be less than 23°C (Muthoni et al., 2012; Almekinders and Struik, 1994); in our study with short-day varieties, the maximum daytime temperature was above 30°C, which likely reduced the duration of inflorescence and flower development.
Fruit and seed development. The SUP and CAP varieties did not form fruits, while ESM formed fruits in less than 25% of the plants during the 35 d of irrigated conditions and the 11 d of the water deficit; SUP and CAP did not form fruits due to the high male sterility (Segura et al., 2006).
Senescence. The senescence, registered from the beginning of the yellowing of the primary leaves and until the leaves and stem were dead, blanched and dry (Meier, 2001), had a different duration for the varieties: 9 d for ESM, 16 d for SUP and 37 d for CAP (Tab. 2, Fig. 2). Segura et al. (2006) reported a senescence duration for leaves and stems of 27 d for ESM and 20 d for CAP and SUP. Senescence can be accelerated by increasing the temperature (Ewing and Struik, 1992), as happened in the present study for ESM and SUP (Fig. 2), where the temperature of the growth stage was 19°C.
Growth and yield parameters
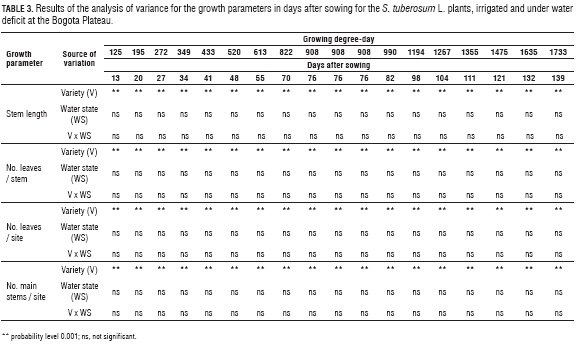
The stem length, the number of leaves per stem and per site and the number of main stems per site showed significant differences (P≤0.01) between the varieties, but not for the two water regimens (Tab. 3). Although it is known that S. tuberosum L. is a species with high drought sensitivity because it has a shallow root system (Liu et al., 2006; Lahou et al., 2006; Ierna and Mauromicale, 2006), the moderate reduction in the growth parameters may have been related to the short duration of the applied water deficit; however, effects of a water stress depends on the variety, state of the ground water potentials were very low for a glycophyte development and duration of the water deficit (Deblonde plant (-1.2 to -1.5 MPa) (Pinheiro et al., 2004, Correia et et al., 2001; Tourneux et al, 2003). al., 2006). It has also been reported that the severity of the effects of a water stress depends on the variety, state of development and duration of the water deficit (Deblonde et al., 2001; Tourneux et al., 2003).
Stem length. The longest stem length was seen in SUP at 121 d (1,475 GDD) and the lower lengths in ESM and CAP (Fig.3). These results are similar to those reported by Ñústez et al. (2009), who found a greater accumulation of dry mass (dry mass) in SUP and a lower one in ESM and CAP. For the three varieties under both water regimens, the model of best fit according to the correlation coefficient (R2> 0.97) for this parameter was the cubic polynomial (Fig. 3).
Leaves per stem and per site. The leaves per stem (Fig. 4) and per site (Fig. 5) did not vary with the water deficit. At 98 d (1,116 GDD), the number of leaves per stem and per site was 16 and 105 for CAP, 15 and 87 for SUP and 12 and 54 for ESM (Fig. 4 and 5). For the number of leaves per stem and per site for the three varieties in the two water states, the model of best fit according to the coefficient of correlation (R2>0.94) was the cubic polynomial (Fig. 4; Fig. 5).
Duration of green foliage. The foliage duration was 132 d for ESM and SUP and 98 d for CAP (Fig. 4; Fig. 5). The length of the growing season in S. tuberosum L. plants should be between 99 and 104 das for early clones and between 123 and 128 dasp for late clones (Allen and Scott, 1980). The rate of appearance of leaves up to 111 d was 0.8 leaves per plant per day for CAP, 0.5 for ESM and 0.7 for SUP.
Main stems per site. The number of main stems was 4.67 for CAP, 4 for SUP and 3 for ESM and did not vary with the water deficit (Fig. 6). In potato, stem morphology is influenced by temperature; at temperatures above 25°C, the branching of the plant (Struik et al., 1989) is reduced. The average daily temperatures of 18.75°C (CAP), 19.95°C (SUP) and 18.79°C (ESM) favored the development of main shoots. For main stems per site, the model of best fit according to the correlation coefficient (R2> 0.80) was the polynomial model for CAP and the exponential model for ESM and SUP (Fig. 6).
Potential tuber yield. The potential tuber yield was decreased by the water deficit by 16.68% for CAP, 15.45% for SUP and 19.46% for ESM, with no statistically significant differences (Fig. 7). It has been reported that S. tuberosum L. is sensitive to water deficits and water availability should not be reduced by more than 30% to achieve optimum yields (Tourneux et al., 2003; Darwish et al., 2006). It has been reported that the limited availability of water during development reduces potato plant growth, yield, number of tubers per plant and tuber size and quality (Karafyllidis et al., 1996; Yuan et al. 2003; Ierna and Mauromicale, 2010). Here, we did not observe an effect of the water deficit, probably because of the short duration of the stress. But, because the leaf water potential reached a negative value, lower than that reported as the permanent wilting point for the potato in some studies, the results suggested that the three studied potato varieties have mechanisms they allow them to tolerate water loss, lowering the leaf water potentials to around -2.0 MPa, which had not been reported for Colombian varieties; the only report was for two Peruvian varieties where a foliar potential of -2.0 MPa had a greater yield reduction than that observed here (Martínez and Moreno, 1992).
The results demonstrated the plasticity of the varieties CAP, SUP and ESM to adjust metabolism in the seasonal drought periods, modifying the phenology. Likewise, the results showed that these varieties have a high response potential to a short-term water-deficit stress, reaching potentials that are very negative for a glycophyte plant without a decrease in the yield.
Acknowledgments
We wish to thank the Vicerrectoria de Investigación of the Pontificia Universidad Javeriana for funding this research with ID code 00004558. To the Graduate Program of the Ciencias Agrarias Faculty of the Universidad Nacional de Colombia for financing part of this study and providing the greenhouse and measuring equipments. Finally, we are grateful to Darwin Moreno, Agronomic Engineer, for his technical assistance during this experiment.
Literature cited
Aksic, M., S. Gudzic, N. Deletic, N. Gudzic, S. Stojkovic, and J. Knezevic. 2014. Tuber yield and evapotranspiration of potato depending on soil matric potential. Bulg. J. Agric. Sci. 20, 122-126. [ Links ]
Allen, E.J. and R.K. Scott. 1980. An analysis of growth of the potato crop. J. Agr. Sci. 94, 583-606. Doi: 10.1017/S0021859600028598. [ Links ]
Almekinders, C.J.M. and P.C. Struik. 1994. Photothermal response of sympodium development and flowering in potato (Solanum tuberosum L.) under controlled conditions. Neth. J. Agri. Sci. 42, 311-329. [ Links ]
Arnold, C.Y. 1959. The determination and significance of base temperature in a linear heat unit system. Proc. Amer. Soc. Hort. Sci. 74, 430-445. [ Links ]
Barrios-Gómez, E.J. and C. López-Castañeda. 2009. Temperatura base y tasa de extensión foliar en frijol. Agrociencia 43, 29-35. [ Links ]
Bonhomme, R. 2000. Bases and limits to using 'degree day' units. Eur. J. Agron. 13, 1-10. Doi: 10.1016/S1161-0301(00)00058-7. [ Links ]
Bouzo, C.A and M.G. Küchen. 2012. Effect oftemperature on melon development rate. Agron. Res. 10, 283-294. [ Links ]
Cao, W. and T.W. Tibbitts. 1995. Leaf emergence on potato stems in relation to thermal time. Agron. J. 87, 474-477. Doi: 10.2134/agronj1995.00021962008700030013x. [ Links ]
Correia, M.J., M.L. Osório, J. Osório, I. Barrote, M. Martins, and M.M. David. 2006. Influence of transient shade periods on the effects of drought on photosynthesis, carbohydrate accumulation and lipid peroxidation in sunflower leaves. Environ. Exp. Bot. 58, 75-84. Doi: 10.1016/j.envexpbot.2005.06.015. [ Links ]
Darwish, T.M., T.W. Atallah., S. Hajhasan, and A. Haidar. 2006. Nitrogen and water use efficiency of fertigated processing potato. Agric. Water Manage. 85, 95-104. Doi: 10.1016/j.agwat.2006.03.012. [ Links ]
Deblonde, P.M.K. and J.F. Ledent. 2001. Effects of moderate drought conditions on green leaf number, stem height, leaf length and tuber yield of potato cultivars. Eur. J. Agron. 14, 31-41. Doi: 10.1016/S1161-0301(00)00081-2. [ Links ]
Desclaux, D. and P. Roumet. 1996. Impact of drought stress on the phenology of two soybean (Glycine max L. Merr) cultivars. Field Crops Res. 46, 61-70. Doi: 10.1016/0378-4290(95)00086-0. [ Links ]
Erwin, J.E and R.D. Heins. 1995. Thermomorphogenic responses in stem and leaf development. HortScience 30, 940-949. [ Links ]
Ewing, E.E. 1981. Heat stress and the tuberization stimulus. Am. Potato J. 58, 31-49. Doi: 10.1007/BF02855378. [ Links ]
Ewing, E.E. and P.C. Struik. 1992. Tuber formation in potato: induction, initiation, and growth. Hortic. Rev. 14, 89-198. Doi: 10.1002/9780470650523.ch3. [ Links ]
Fedepapa. 2004. Guía ambiental para el cultivo de la papa. Ministerio de Ambiente, Vivienda y Desarrollo Territorial, Bogotá [ Links ].
Flores-López, R., F. Sánchez-del Castillo, J.E. Rodríguez-Pérez, R. Mora-Aguilar, M.T. Colinas-León and H. Lozoya-Saldaña. 2009. Influencia de la radiación solar en la producción de semilla-tubérculo de papa bajo cultivo sin suelo. Rev. Chapingo Ser. Hortic. 15, 25-30. [ Links ]
Goeser, N.J., P.D. Mitchell, P. D. Esker, D. Curwen, G. Weis, and A.J. Bussan. 2012. Modeling long-term trends in russet burbank potato growth and development in Wisconsin. Agron. 2, 14-27. Doi: 10.3390/agronomy2010014. [ Links ]
Gutiérrez, J.R., A. Bravo, N. Jaeger, and E.R. Hajek. 1985. Fenología de la papa (Solanum tuberosum) y su relación con la temperatura en Lipingüe (Décima Región, Chile). Cienc. Invest. Agrar. 12, 137-142. [ Links ]
Hossain, A., J.A.Teixeira da Silva., M.V. Lozovskaya, and V. P. Zvolinsky. 2012. The effect of high temperature stress on the phenology, growth and yield of five wheat (Triticum aestivum L.) genotypes. Asian Australas. J. Plant Sci. Biotechnol. 6, 14-23. [ Links ]
Ierna, A. and G. Mauromicale. 2006. Physiological and growth response to moderate water deficit of off-season potatoes in a Mediterranean environment. Agric. Water Manage. 82, 193-209. Doi: 10.1016/j.agwat.2005.05.005. [ Links ]
Jensen, C.R., A. Battilani, F. Plauborg, G. Psarras, K. Chartzoulakis, F. Janowiak, R. Stikic. Z. Jovanovic, G. Li, X. Qi, F. Liu, S.-E. Jacobsen, and M.N. Andersen. 2010. Deficit irrigation based on drought tolerance and root signalling in potatoes and tomatoes. Agric. Water Manage. 98, 403-413. Doi: 10.1016/j. agwat.2010.10.018. [ Links ]
Juskiw, P.E., Y.W. Jame, and L. Kryzanowski. 2001. Phenological development of spring barley in a short-season growing area. Agron. J. 93, 370-379. [ Links ]
Karafyllidis, D.I,, N. Stavropoulos, and D. Georgakis. 1996. The effect of water stress on the yielding capacity of potato crops and subsequent performance of seed tubers. Potato Res. 39, 153-163. Doi: 10.1007/BF02358215. [ Links ]
Kawakami, J., K. Iwama, and Y. Jitsuyama. 2006. Soil water stress and the growth and yield of potato plants grown from microtubers and conventional seed tubers. Field Crops Res. 95, 89-96. Doi: 10.1016/j.fcr.2005.02.004. [ Links ]
King, B.A., J.C. Stark, and S.L. Love. 2004. Potato production with limited water supply. CIS1122. Idaho Agricultural Extension Service, University of Idaho, Moscow, ID. [ Links ]
Körner, C. and D. Basler. 2010. Phenology under global warming. Science 327, 1461-1462. Doi: 10.1126/science.1186473. [ Links ]
Kuiper, H.A., G.A. Kleter., H.P. Noteborn and E.J. Kok. 2001. Assessment of the food safety issues related to genetically modified foods. Plant J. 27, 503-528. [ Links ]
Kuppinger, L., J. Auber, E. Farfan, M.A. Khan, M. Bonierbale, and F. Asch. 2014. Effects of drought stress on crop development, growth and chlorophyll fluorescence in five potato clones. p. 54. In: Tielkes, E. (ed.). Bridging the gap between increasing knowledge and decreasing resources. Czech University of Life Sciences, Prague. [ Links ]
Lahlou, O., S. Ouattar, and J.F. Ledent. 2003. The effect of drought and cultivar on growth parameters, yield and yield components of potato. Agronomie 23, 257-268. Doi: 10.1051/agro:2002089. [ Links ]
Lilley, J.M and S. Fukai. 1994. Effect of timing and severity of water deficit on four diverse rice cultivars III. Phenological development, crop growth and grain yield. Field Crops Res. 37(3), 225-234. [ Links ]
Liu, F., C.R. Jensen, A. Shahanzari, and M.N. Andersen, and S.-E. Jacobsen. 2005. ABA regulated stomatal control and photo-synthetic water use efficiency of potato (Solanum tuberosum L.) during progressive soil drying. Plant Sci. 168, 831-836. Doi: 10.1016/j.plantsci.2004.10.016. [ Links ]
Liu, F., A. Shahnazari., M.N. Andersen., S.E. Jacobsen, and C.R. Jensen. 2006. Effects of deficit irrigation (DI) and partial root drying (PRD) on gas exchange, biomass partitioning, and water use efficiency in potato. Sci. Hortic. 109,113-117. Doi: 10.1016/j.scienta.2006.04.004. [ Links ]
Martínez, C.A and U. Moreno. 1992. Expresiones fisiológicas de resistencia a la sequía en dos variedades de papa sometidas a estrés hídrico en condiciones de campo. R. Bras. Physiol. Veg. 4, 33-38. [ Links ]
Mazurczyk, W., B. Lutomirska, and A. Wierzbicka. 2003. Relation between air temperature and length of vegetation period of potato crops. Agric. Forest Meteorol. 118, 169-172. Doi: 10.1016/S0168-1923(03)00113-8. [ Links ]
McDowell, N.G., W.T. Pockman, C.D. Allen., D.D. Breshears, N. Cobb, T. Kolb, J. Plaut, J.S. Sperry, A. West, D.G. Williams, and E.A. Yepez. 2008. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol. 178, 719-739. [ Links ]
Meier, U. 2001. Estadios de las plantas mono-y dicotiledóneas. BBCH Monografia. 2nd ed. Centro Federal de Investigaciones Biológicas para Agricultura y Silvicultura, Berlin. [ Links ]
Morales, C.G., M.T. Pino, and A. del Pozo. 2013. Phenological and physiological responses to drought stress and subsequent re-hydration cycles in two raspberry cultivars. Sci. Hortic. 162, 234-241. Doi: 10.1016/j.scienta.2013.07.025. [ Links ]
Muthoni, J., H. Shimelis., R. Melis, and J. Kabira. 2012. Reproductive biology and early generation's selection in conventional potato breeding. Aust. J. Crops Sci. 6, 488-497. [ Links ]
Ñústez L., C., M. Santos C., and M. Segura A. 2009. Acumulación y distribución de materia seca de cuatro variedades de papa (Solanum tuberosum L.) en Zipaquirá, Cundinamarca (Colombia). Rev. Fac. Nal. Agr. Medellin 62, 4823-4834. [ Links ]
Ñústez, C.E. 2011. Variedades colombianas de papa. Faculty of Agronomy, Universidad Nacional de Colombia, Bogotá [ Links ].
Oki, T. and S. Kanae. 2006. Global hydrological cycles and world resources. Sciences 313, 1068-1072. Doi: 10.1126/science.1128845. [ Links ]
Pinheiro, C., J.A. Passarinho, and C.P. Ricardo. 2004. Effect of drought and rewatering on the metabolism of Lupinus albus organs. J. Plant Physiol. 161, 1203-1210. [ Links ]
R Development Core Team. 2010. A language and environment for statistical computing. R Foundation for Statistical Computing, Viena. [ Links ]
Rolando, J.L., D.A. Ramírez, W. Yactayo, P. Monneveux, and R. Quiroz. 2015. Leaf greenness as a drought tolerance related trait in potato (Solanum tuberosum L.). Environ. Exp. Bot. 110, 27-35. Doi: 10.1016/j.envexpbot.2014.09.006. [ Links ]
Sale, P.J.M. 1979. Growth of potatoes (Solanum tuberosum L.) to the small tubers stage as related to soil temperature. Aust. J. Agric. Res. 30, 667-675. [ Links ]
Sarquís, J.I., H. González, and I. Bernal-Lugo. 1996. Response of two potato clones (S. tuberosumL.) to contrasting temperature regimes in the field. Am. Potato Res. 73, 285-300. Doi: 10.1007/BF02855207. [ Links ]
Segura, M.A., M.C. Santos, and C.E. Ñústez. 2006. Desarrollo fenológico de cuatro variedades de papa (Solanum tuberosum L.) en el municipio de Zipaquirá (Cundinamarca). Fitotecnia Colomb. 6, 33-43. [ Links ]
Struik, P.C., J. Geertsema, and C.H.M.G. Custers. 1989. Effects of shoot, root and stolon temperature on the development of the potato (Solanum tuberosum L.) plant. III. Development of tubers. Potato Res. 32, 151-158. Doi: 10.1007/BF02358227. [ Links ]
Teixeira, J. and S. Pereira. 2007. High salinity and drought act on an organ-dependent manner on potato glutamine synthetase expression and accumulation. Environ. Exp. Bot. 60, 121-126. Doi: 10.1016/j.envexpbot.2006.09.003. [ Links ]
Timlin, D., S.M.L. Rahman, J. Baker, V.R. Reddy, D. Fleisher, and B. Quebedeaux. 2006. Whole plant photosynthesis, development, and carbon partitioning in potato as a function of temperature. Agron. J. 98, 1195-1203. Doi: 10.2134/agronj2005.0260. [ Links ]
Touchette, B.W., G.A. Smith, K.L. Rhodes, and M. Poole. 2009. Tolerance and avoidance: Two contrasting physiological responses to salt stress in mature marsh halophytes Juncus roemerianus Scheele and Spartina alterniflora Loisel. J. Exp. Marine Biol. Ecol. 380, 106-112. Doi: 10.1016/j.jembe.2009.08.015. [ Links ]
Tourneux, C., A. Devaux, M.R. Camacho, I.P. Mamani, and J.-F. Le-dent. 2003. Effects ofwater shortage on six potato genotypes in the highlands of Bolivia (II): water relations, physiological parameters. Agronomie 23, 180-190. Doi: 10.1051/agro:2002080. [ Links ]
Van den Bilcke, N., D.J. Simbo, and R. Samson. 2013. Water relations and drought tolerance of young African tamarind (Tamarindus indica L.) trees. S. Afr. J. Bot. 88, 352-360. Doi: 10.1016/j.sajb.2013.09.002. [ Links ]
Van Loon, C.D. 1981. The effect of water stress on potato growth, development and yield. Am. Potato J. 58, 51-69. Doi: 10.1007/BF02855380. [ Links ]
Villaseca, S., H. Guglielmetti, and R. Novoa. 1988. Fenología y sumas térmicas en papa. Simiente 58, 10. [ Links ]
Vos, J. and A.J. Haverkort. 2007. Water availability and potato crop performance. pp. 333- 351. In: Vreugdenhil, D., J. Bradshaw, C. Gebhardt, F. Govers, D.K.L. Mackerron, M.A. Taylor, and H.A. Ross (eds.). Potato biology and biotechnology: advances and perspectives. Elsevier, Amsterdam, The Netherlands. Doi: 10.1016/B978-044451018-1/50058-0. [ Links ]
Wang, Y., J. Jiang, X. Zhao, G. Liu., C. Yang, and L. Zhan. 2006. A novel lea gene from Tamarix androssowii confers drought tolerance in transgenic tobacco. Plant Sci. 171, 655-662. Doi: 10.1016/j.plantsci.2006.06.011. [ Links ]
Wang, D., Y. Kang, and S. Wan. 2007. Effect of soil matric potential on tomato yield and water use under drip irrigation condition. Agr. Water Manage. 87, 180-186. Doi: 10.1016/j.agwat.2006.06.021. [ Links ]
Yamasaki, T., T. Yamakawa, Y. Yamane, H. Koike, K. Satoh, and S. Katoh. 2002. Temperature acclimation of photosynthesis and related changes in photosystem II electron transport in winter wheat. Plant Physiol. 128, 1087-1097. Doi: 10.1104/pp.010919. [ Links ]
Yuan, B.Z., S. Nishiyama, and Y. Kang. 2003. Effects of different irrigation regimes on the growth and yield of drip-irrigated potato. Agr. Water Manage. 63, 153-167. Doi: 10.1016/S0378-3774(03)00174-4. [ Links ]
Yuan, F.M. and W.L. Bland. 2004. Light and temperature modulated expolinear growth model for potato (Solanum tuberosum L.). Agr. Forest Meteorol. 121, 141-151. Doi: 10.1016/j.agrformet.2003.08.032. [ Links ]
Zegada-Lizarazu, W. and A. Monti. 2013. Photosynthetic response of sweet sorghum to drought and re-watering at different growth stages. Physiol. Plant. 149, 56-66. Doi: 10.1111/ppl.12016. [ Links ]