INTRODUCTION
One of the purposes of endodontic treatment is the elimination of pulp and periapical infections by disinfecting and sealing the root canal system. The use of gutta-percha and a sealing cement is generally suggested to achieve a three-dimensional sealing with a cement provided with bactericidal characteristics that inhibit the growth of residual microorganisms in the root canal, favoring a successful treatment outcome.1-2) Multiple predictor factors alter the success of an endodontic treatment, becoming a real challenge for endodontists; some of the most common include, but are not limited to: anatomical complexity of the canal system (classification of root canal system according to Vertucci-Sert and Bayirli)3-4 and the presence of branches of the main root canal (apical deltas), especially in the root’s apical third; periodontal pockets indicating endodontic-periodontal compromise in the tooth; the preoperative state of pulp tissue; the presence or absence of an apical lesion and the size of it (initial pulp and periapical diagnosis); incorrect postoperative restoration; the number of appointments required for the treatment; the obtained working length; the root canals omitted during the procedure, and whether this is the first time an endodontic treatment is performed, or is a retreatment. 5-8
The persistence of microorganisms within the root canal system is the main cause of endodontic failure.6-11 The root canal offers ideal conditions (like a microenvironment with abundant nutrients, ideal pH, and the presence of spaces that favor the development of biofilms, like dentinal tubules and apical deltas) for bacteria to evade the mechanical effects produced by limes and the chemical effects of irrigants or medications. Persistent bacteria can survive for many years, being able to colonize, form biofilms and induce tissue re-inflammation.12 Enterococcus faecalis (E. faecalis) is the microorganism most frequently found in endodontically treated root canals,12 being the most common cause of endodontic failure, with a prevalence of 90%. It can easily penetrate dentinal tubules, where it escapes the action of medicines and instruments, resists high pH levels, lives with or without nutrients available, and enters a viable state when exposed to unfavorable environmental conditions.13-14 Endodontic failure is a problem affecting a high percentage of the treated population (6-20%); it has been shown that the techniques, materials, and instruments currently used have not been able to achieve entire success of endodontic therapy, still with high failure rates.1,15,16 The search for new alternatives to ensure 100% success in endodontic treatments is still a current need.
Nanotechnology appears as a potential alternative in the field of endodontics to provide a solution to this situation, though it has not yet been sufficiently evaluated or studied in this field. It is defined as the “science of material manipulation at the billionth of a meter or nanometer scale, roughly the size of two or three atoms”, studying particles with measures ranging from 1 to 100 nm.17-18
Many types of sealers have been introduced in endodontics over the years, seeking to improve the characteristics proposed by Grossman.19 While zinc oxide-eugenol fails to comply with all the suggested characteristics, it has been widely used in endodontic therapy for more than 70 years with good clinical results.20 It consists eugenol in Colombia is Grossfar cement (Laboratorio Eufar, Bogotá, Colombia), whose manufacturer has not provided a well defined mixing ratio, which may influence setting time and possibly cause cytotoxicity in the host’s tissues.21
Since ancient times, silver has been widely used for its antimicrobial properties in traditional medicine. In mid-20th-century dentistry, it was used to manufacture root canal sealing cones,19,20 but these are not used anymore because of their several disadvantages, including oxidation followed by pigmentation of the dental structure; in addition, they could not be molded to seal the root canal walls in 360°, reach the apical end, and therefore were not used very often in teeth with irregular shapes or in curved and narrow canals.19-23 Silver has also been added into various dental compounds, such as composite resins, toothpastes, chlorhexidine, glass ionomer and MTA (Mineral Trioxide Aggregate).24,25 Its toxicity depends on aspects like particle size, shape, surface area and load, solubility, and agglomeration state. It has been shown that when used at a nano scale, its toxicity levels and ability to pigment the structure with which it is in contact are low.22
Multiple studies in various fields show that nanomaterials have a high potential to decrease the formation of bacterial biofilm, inhibiting the demineralization process.26 Therefore, its potential bonding with dental materials has been studied, enhancing their antimicrobial power as well as their characteristics in order to find the best solution for the management of oral pathologies.24,27
The purpose of this study is to evaluate the antimicrobial capacity of silver nanoparticles immobilized in a zinc oxide-eugenol cement against E. faecalis in order to improve the prognosis of endodontic treatments.
METHODS
This was an in vitro study with E. faecalis strain ATCC 29212 as the unit of analysis and perfomed under the approval of the Universidad CES’ Ethics Committee, which classified the project as a risk I study, according to standard 8430 of October 4, 1993.28
The synthesis of silver nanoparticles was performed following the protocol of the Biomaterials Laboratory (Universidad EIA and Universidad CES, Sabaneta, Antioquia, Colombia) (Figure 1).

Figure 1 Synthesis of solid silver nanoparticles and suspension by obtaining tree tomato and guava extracts. The color change of the solution over time indicates the presence of nanoparticles in it.
UV-visible spectroscopy was conducted using the Thermo Scientific NanoDrop 2000 Spectrophotometer (Wilmington, United States) (absorbance analysis between 190 and 840 nm) to confirm the presence of silver nanoparticles in the prepared solutions.
The zinc oxide-eugenol mixture was standardized because the cement used in this study, Grossfar Cement (Laboratorio Eufar, Bogotá, Colombia), does not currently indicate the correct and accurate proportion for use. If varying proportions are used, it risks causing changes in the properties of the material. Sample homogeneity must be ensured, and the same amount of powder and liquid should always be used in all the samples to be generated. Standardization was conducted by a single operator in an extraction cabinet (ESCO-Serie E Changi, Singapore) at a temperature of 36 °C to simulate the characteristics of the mouth. The zinc oxide was weighed and the eugenol volume measured, in such a way that a drop consistency was obtained when mixed. The setting time was taken from the moment the mixture was made until it hardened. The mixture was made in triplicate, always using the same amounts of powder and liquid. All samples reached the same setting time without any change in material characteristics.
To prepare the integrated material (zinc oxide-eugenol, silver nanoparticles), tests were conducted in an extraction cabinet at a temperature of 36 °C and 65% relative humidity. Once the mixture of silver nanoparticles with zinc eugenol-oxide was obtained, the setting time was calculated. Each sample was done in triplicate. The nanoparticles added to the cement do not alter the properties of the material when performed under conditions like those found in the mouth.
Once the samples were set, the material was characterized to ensure that nanoparticles were immersed in the zinc oxide-eugenol mix and evenly distributed in it. The obtained samples of zinc oxide-eugenol cement alone and with silver nanoparticles added were analyzed by scanning electron microscopy (SEM) (JEOL JSM 6490 LV, Beijing, China) with increases of 20μm, 3000x, and Fourier-Transform Infrared Spectroscopy (FTIR) (Perkin Elmer Spectrum 100 Spectrophotometer, Beaconsfield, United Kingdom). Both methods have been respectively recommended for the analysis of morphology of materials and the qualitative/quantitative analysis of functional groups.29,30
Once the material was integrated and characterized, the Kirby-Bauer test was conducted. The ATCC 29212 strain of E. faecalis was activated in blood agar. Three colonies were diluted in a medium of brain heart infusion (BHI) at a concentration equivalent to the 0.5 McFarland standard.31 The Müller-Hinton (MH) agar plates supplied by the Colombian Institute for Tropical Medicine (Universidad CES, Sabaneta, Antioquia, Colombia) were prepared and inoculated. Once the dishes for all assemblies were inoculated, the test was performed at 3 different times with 3 repetitions (24 samples per time) using each of the following samples: zinc oxide-eugenol, zinc oxide-eugenol with silver nanoparticles in suspension of tree tomato extract, zinc oxide-eugenol with silver nanoparticles suspended in guava extract, zinc oxide-eugenol with solid silver nanoparticles, silver nanoparticles in tree tomato extract suspension, silver nanoparticles in guava extract suspension, positive control (penicillin G), negative control (saline solution). The dishes were read 24 hours after the assembly.
The data were recorded in an Excel® spreadsheet and finally consolidated and exported to the Stata software.
RESULTS
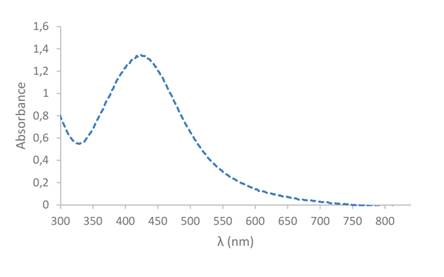
Figures 2 and 3 show the results of the UV- visible spectroscopy analysis for nanoparticle solutions obtained from guava and tree tomato extracts. The maximum absorbance peak occurs at about 418nm in the guava solution and 440nm in the tree tomato solution. These peaks are characteristic of the presence of silver nanoparticles and are explained by the surface plasmons phenomenon produced in the interaction of metal nanoparticles with the electromagnetic field of light.32,33

Figure 4 (A-C): Images obtained by SEM at 20um, 3.000x. A. Zinc oxide-eugenol. B. Zinc oxide-eugenol + nanoparticles in suspension. The arrow shows the presence of silver nanoparticles in the material. C. Zinc oxide-eugenol + solid nanoparticles. The arrow shows the presence of solid silver nanoparticles immersed in the material.
The morphology of the samples observed by SEM showed a detailed view of the internal structures and the distribution of the nanoparticles within the cement (Figure 4).
These findings suggest that silver nanoparticles in both solid and suspended forms are integrated into the zinc oxide-eugenol system.
The results of the FTIR spectra are shown in Figures 5, 6 and 7.
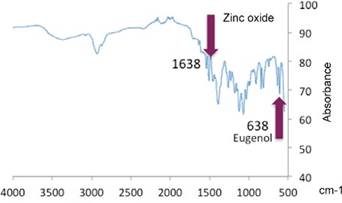
Representative bands: 1,641 cm-1 and 620 cm-1: deformation tensions and vibrations of zinc oxide and eugenol respectively. 1,205 cm-1-OH (flat deformation) of zinc oxide. 1,124 cm-1- OH Tension mainly due to absorption of water from zinc oxide. 1,035 cm-1 C-H Tension vibration of the benzene ring of zinc oxide. 420cm-1 - 480 cm-1 Eugenol tension vibration. The results show the same bands as the reference samples of zinc oxide-eugenol alone (bands: 1,641 cm-1 zinc oxide and 620 cm-1 eugenol).34,35
In Figures 6 and 7, the bands in 1,638 cm-1 zinc oxide and 638 cm-1 eugenol are displaced, suggesting that the presence of nanoparticles modified the positions of those groups. The created material shows presence of silver nanoparticles both in suspension and in their solid form.
Figure 8 shows the inhibition halos formed during the Kirby-Bauer test and Table 1 shows the halos measures averages.

Figure 8 (A-C). Inhibition halos: Inhibition halos: A. Zinc oxide-eugenol. B. Zinc oxide-eugenol with silver nanoparticles in tree tomato extract. C. Silver nanoparticles in suspension in tree tomato extract. D. Positive control, Penicillin G. E. Negative control, saline solution. F. Zinc oxide-eugenol with nanoparticles suspended in guava extract. G. Zinc oxide-eugenol with solid nanoparticles. H. Nanoparticles suspended in guava extract
Table 1 Average of inhibition halos measures
| Sample | Average halo (μm) |
|---|---|
| Positive control | 7838.90 |
| Negative control | 0 |
| ZOE | 4,313.22 |
| ZOE+NPs suspension (tree tomato) | 4,137.10 |
| ZOE+NPs suspension (guava) | 4,366.58 |
| ZOE+solid NPs | 4,528.80 |
| NPs suspension (guava) | 7,257.09 |
| NPs suspension (tree tomato) | 4,896.08 |
ZOE: Zinc Oxide-Eugenol
NPs: Silver Nanoparticles
The Shapiro-France test was conducted, rejecting the hypothesis of normality for data distribution. A Kruskal-Wallis test was performed, rejecting the hypothesis (p = 0.0045 and p = 0,0345). At least one of the groups has significant differences with the others. The group of nanoparticles suspended in guava extract has a much higher sum of ranks than the others. An ANOVA + Scheffé test was then performed in order to better observe the groups and identify the differences among them. The group of nanoparticles suspended in guava extract has significant differences with all the other groups, except with the group of nanoparticles suspended in tree tomato extract, in which there is a high difference among the averages, but this difference is not significant (p > 0,05).
DISCUSSION
Setting time is the time required for the sealant to reach its final properties; this depends on variables like components, particle size, room temperature, and relative humidity.28,29 Sealing cements have no standard setting time, but this should be long enough to allow the cement’s application and fitting as a sealer inside the root canal.30 According to the manufacturer, zinc oxide-eugenol cement hardens extra-orally after 2 hours at 37 °C and 100% humidity; however, the exact setting time depends on aspects such as handling technique, consistency, humidity and temperature, and moistness of the glass tile and spatula.31
As this study found out, when all samples were processed -including the zinc oxide-eugenol control group-, and once the working times were compared against samples with silver nanoparticles, there were no significant differences in setting time, suggesting that there is no change in final preparation time and that this variable is not affected at the time of manufacture. However, when comparing the times in this study with those reported in the literature (2 hours), there is a setting time increase in the created cement, with 4 hours and 35 minutes for the sample of zinc oxide-eugenol plus nanoparticles in suspension, and 4 hours 25 minutes for the sample of zinc oxide-eugenol plus solid nanoparticles. This change may be due to the aforementioned variables (handling technique, consistency, humidity and temperature, moisture of the glass tile and spatula), but it is not a significant change.
Samples created from zinc oxide-eugenol cement alone and with silver nanoparticles were analyzed using SEM and infrared spectrometry. The morphologies found in our study for zinc oxide-eugenol cement are similar to those reported in 1986 by Bayne et al, who analyzed this compound through SEM to determine its morphological characteristics.32) These results were confirmed by Gaspar et al in 2017,33 so it can be concluded that the manufactured cement’s morphological characteristics are similar to those of the cement reported in the literature, and it is expected to have the same properties.
Infrared spectrometry was used for characterization of the material in order to determine whether silver nanoparticles were immersed in the created material by analyzing functional groups and their positions. The results show the same bands as those found by Mazinis et al in 200734 and Dhayagude et al in 201735 for zinc oxide-eugenol alone (1,641 cm-1 zinc oxide and 620 cm-1 eugenol). These bands are displaced (1,638 cm-1 zinc oxide and 638 cm-1 eugenol) because of the presence of a different material within the compound -silver nanoparticles in this case-. It can be concluded that silver nanoparticles are present in the created material.
E.faecalis was used in this study because it is one of the most resistant microorganisms, largely associated with endodontic failure.34 This microorganism can evade conventional endodontic disinfection systems, by hiding in branches of the root canal and denti tubules. High fluidity and penetration of a sealing cement with antibacterial properties are ideal conditions for removing these microorganisms.35 Zinc oxide-eugenol is widely used as an endodontic sealing cement.36 This cement showed bacterial growth inhibition in the present study, with inhibition halos in all the evaluated samples (4,313.22 µm average). This result is comparable to another study that used three endodontic sealants against E. faecalis, all of which showed inhibition of the microorganism.37
Silver is known to be an antimicrobial agent that increases its bactericidal potential when reduced to a nanoscale.38 The halos observed in the present study confirm the antimicrobial action of silver nanoparticles- in this case against E. faecalis-, similar to the findings by Wu et al,39 in which the microorganism was eliminated after 7 days of contact with the silver nanoparticle gel, used as intracanal medication. The results differ when an irrigation agent is used, as it remains in contact with the microorganism for only 2 minutes, with no significant change in biofilm. The authors conclude that a minimum contact time between microorganism and silver nanoparticles is required in order to successfully remove the microorganism. Our study shows the presence of an inhibition halo 24 hours after contact of the microorganism with silver nanoparticles.
A similar study conducted by Hahgoo et al showed that there is no significant difference between zinc oxide-eugenol cement mixed with silver nanoparticles at different concentrations (0.5, 2 and 5%) and the original cement when it comes to antibacterial capacity against E. Faecalis.40 Our study shows that the combination of silver nanoparticles with zinc oxide eugenol cement results in inhibition of the microorganism, regardless of the nanoparticles being solid or in suspension. The inhibition halo is smaller in samples containing zinc oxide-eugenol cement plus suspended nanoparticles compared to samples with zinc oxide-eugenol cement plus solid nanoparticles, but their averages are not statistically significant. Suspended nanoparticles have a higher degree of diffusion in agar, allowing for higher inhibition when used alone, but by bonding them with zinc oxide-eugenol cement, they have a larger trajectory to cross for their diffusion process, so their effect is reduced and does not enable the formation of a larger inhibition halo. When guava extract is used, the inhibition halo is larger; this may be because guava particles are smaller, with more particles per square millimeter, so their antibacterial capacity may be higher. When mixed with zinc oxide-eugenol, solid silver nanoparticles are apparently able to get closer to the surface of the compound, enabling a larger diffusion and therefore a larger average in inhibition halos. Apparently, the best antimicrobial activity occurs with the bonding of zinc oxide-eugenol and nanoparticles in solid state. Obtaining solid nanoparticles requires an additional freeze-dried step, which is highly demanding in terms of both time and equipment, and the amount obtained is very limited, making the process very expensive and impractical.
CONCLUSIONS
Zinc oxide-eugenol cement was integrated with created silver nanoparticles. The integrated material has antimicrobial properties against E. faecalis but these are not statistically significant with respect to the antimicrobial activity of zinc oxide-eugenol cement. Silver nanoparticles in guava extract suspension have statistically significant differences in their bactericidal activity compared to the other samples, so this is a starting point for improving the characteristics of the zinc oxide-eugenol compound.