Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.15 no.1 Medellín Jan./June 2008
FARMACOLOGY AND TOXICOLOGY
HALLAZGOS COMPLEMENTARIOS SOBRE LA ACTIVIDAD ANTIMALÁRICA DEL AZUL DE METILENO
Y SU TOXICIDAD
COMPLEMENTARY FINDINGS ON THE ANTIMALARIAL ACTIVITY AND TOXICITY OF METHYLENE BLUE.
Giovanny GARAVITO.1*; Stephane BERTANI.2; Eric DEHARO.3
1 Departamento de Farmacia, Facultad de Ciencias, Universidad Nacional de Colombia (DFUNC), Carrera 30 45-03, Bogotá D.C. Colombia
2 Laboratoire de Parasitologie Comparée et Modèles Expérimentaux (USM0307) Muséum National d'Histoire Naturelle (MNHN), 61, rue Buffon, 75231 Paris Cedex 05, France;
3 UMR-152 IRD – Université Toulouse 3, Paul Sabatier, Faculté des Sciences Pharmaceutiques, 31062 Toulouse cedex 9, France
Resumen
En 1881, Ehrlich reportó el azul de metileno como el primer antimalárico sintético, pero dejo de ser empleado con este propósito; el debe ser reconsiderado pues son necesarias con urgencia nuevas alternativas de bajo costo, en el arsenal de medicamentos antimaláricos. En este trabajo la actividad antimalárica del azul de metileno es evaluada in vivo frente a parásitos de malaria murina. Una dosis de 15 mg/kg/día inhibe el 50% del crecimiento eritrocítico parasitario de Plasmodium berghei y P. yoelii nigeriensis, en tanto que es prácticamente inactivo frente a los estadios hepáticos de P. yoelii yoelii. Sobre un cultivo de la línea celular linfoblástica BJAB, el azul de metileno es 20 veces más citotóxico que la cloroquina, no obstante el azul de metileno presenta valores de índice de selectividad del mismo nivel que la cloroquina, frente a varias cepas de P. falciparum de diferentes niveles de sensibilidad y provenientes de diferentes lugares geográficos.
Palabras clave: Antipalúdicos - Plasmodium - Malaria - Azul de metileno
Abstract
Methilene blue was reported as the first synthetic antimalarial by Ehrlich in 1881. It is currently no longer used for that purpose but it should be reconsidered since new economic alternatives are urgently needed in the arsenal of antimalarial drugs. The antimalarial activity of methylene blue is investigated here in vivo against rodent malaria parasites. 15 mg/kg daily dose of methylene blue inhibits 50% of the erythrocytic parasite growth of Plasmodium berghei and P. yoelii nigeriensis, while on hepatic stages of P. yoelii yoelii is almost inactive. In cell culture experiments with the lymphoblast-like BJAB cells line, it is 20 times more cytotoxic than chloroquine. Nevertheless, methylene blue shows a similar selectivity index as chloroquine against strains of different level of sensitivity of P. falciparum original from different geographical areas.
Keywords: Antimalarials, Plasmodium, Malaria, Methylene blue.
INTRODUCTION
About 3 billion people live in endemic areas where malaria is one of the major health problems. 500 million cases are registered annually, leading to the death of 1,5 million people, most of them children (1). Control measures are largely based on vector eradication programs and clinical treatment, but their efficacy is dramatically reduced by increasing the resistance of vector and parasite. New drugs at low cost are urgently needed. Unfortunately, although the World Health Organization negotiates with pharmaceutical companies in order to reduce the prices of antimalarial drugs, these remain unattainable for most of the affected populations (2). Therefore new and affordable treatments are a major priority for affected countries. In this context, Methylene blue (MB), which has been proven to be active (3, 4, 5) and inexpensive, could be a suitable alternative.
Methylene blue is the oldest and cheapest synthetic drug ever used in antimalarial therapy (Figure 1). In 1891 Guttmann and Ehrlich published the first report of its antimalarial activity (4). Two years later, Ferreira (5) recommended the use of MB by oral route in children with malaria. At the beginning of the 20th century, MB served as structural starting point for the development of 8-aminoquinolines (3, 6, 7) and disappeared thereafter from the antimalarial arsenal. Afterwards, few works on the antimalarial properties of MB have been reported because it was thought to enhance haemolysis in glucose-6-phosphate-deshydrogenase (G6PD) deficiency (8) and could stain the tissues of MB-treated people (9). Recently Mandi et al. (10) and Meissner et al. (11) showed that G6PD deficiency is not commited by the use of MB for malaria treatment, even in class III G6PD deficient young children. These observations renewed the interest of MB as a cost-effective alternative in the treatment of malaria.
This report presents data on the in vivo antimalarial activity of MB against blood and liver forms of rodent malaria parasites and the in vitro cytotoxicity on BJAB cells, thereby completing other studies published by our research team. (12, 13).
MATERIALS AND METHODS
In vivo antimalarial activity
In vivo studies were conducted according to the French and Colombian legislations on laboratory animal use and care (N°2001-464 and N° 008430, respectively).
Effect on the intraerythrocytic cycle
The classical 4-day suppressive test (14) was conducted with P. yoelii nigeriensis or P. berghei ANKA. Swiss female mice (Bioterio DFUNC and Charles River France) weighing 20 ± 2 g, were infected with 107 parasitized cells in 0,9% saline solution (day 0). Batches of five mices were orally treated with MB at doses of 50, 25, 12.5 and 6.35 mg/Kg/day, two hours after infection and at the same hour during 4 consecutive days. A control group received 0,9% saline solution while a reference group was administered chloroquine diphosphate (CQ) at 3 mg/kg/day (oral route). The survival of mice was checked daily and the percentage of parasitized erythrocytes was determined on day 4, by Giemsa-stained thin blood smears from peripheral blood. The percentage of inhibition of parasitaemia was calculated, and the Effective Dose 50 (ED50) was determined with a linear least square regression analysis.
Effect on the hepatic stage
The in vivo activity on the pre-erythrocytic stage was determined according to Peters and Robinson (15): Batches of three mice were intravenously inoculated with 108 sporozoites of P. yoelii yoelii (17X strain; clone 1,1) in 0,9% saline. The treatment (around twice the in vivo determined ED50) was given to mice by oral route, 2 h after inoculation; Primaquine at 60 mg/kg was administered to a reference group. The suppressive effect was estimated on 48, 72, 96, 120, 144, and 168 hours after inoculation by Giemsa-stained thin blood smears inspection. Animals which showing no signs of infection on day 14 were considered as cured.
In vitro studies on BJAB cells
Culture of lymphoblast-like line BJAB cells was carried out on 2 mM L-glutamine-enriched RPMI supplemented with 1ml/L of 10mg/ml gentamycin and 10% heat-inactivated foetal bovine serum at 37°C in 5% CO2 environment. BJAB cells at 2×105 were exposed to serial dilutions of methylene blue for 24 h in culture conditions (test in triplicate). 24 h after initial cell-plating, the cell growth was estimated by [3H]-thymidine incorporation (2,5 µCi/ml in culture medium) for 16 h. Incorporated [3H]-thymidine was then determined with a beta-counter (1450-Microbeta Trilux, Wallac-PerkinElmer). The CC50 (Cytotoxic Concentration 50 i.e. concentration that kills 50% of cultured cells), was determined with a linear least square regression analysis. A control of viability was carried out in parallel.
Reagents
All chemicals reagents were provided by Sigma-Aldrich. Radiolabels were provided by PerkinElmer (Courtaboeuf, France).
RESULTS AND DISCUSSION
Against erythrocytic forms in rodent malaria parasites, MB was more active on P. berghei than on P. yoelii (ED50 of 12,8 ± 8 mg/kg/day and
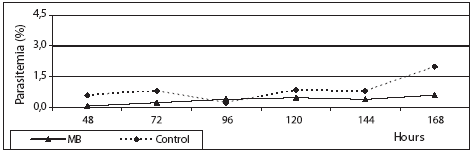
19,4 ± 4 mg/kg/day, respectively) with no signs of acute toxicity after a 4 days treatment (Table 1, Figure 2). MB antimalarial activities were ten times lower than those observed with CQ, that showed ED50 of 0,53 and 1,21 mg/kg/day against P. berghei and P. yoelii nigeriensis, respectively. A previous study reported that MB had ED50 of 11,03 and 1,21 mg/kg/day against P. yoelii nigeriensis and P. vinckei petteri, respectively (3). The low ED50 found with P. vinckei can be explained by the degree of synchronicity of the infection, being P. vinckei more sensitive to antimalarial agents than other rodent malaria species (16). On the contrary, any activity against hepatic stages of P. yoelii yoelii (pre-erythrocytic stage) with a dose of up to 100 mg/kg was not observed (figure 3). This was the expected result since the main action of MB is based on the inhibition of heme-GSH degradation (13), a process not occurring during the pre-erythrocytic cycle of Plasmodium yoelii.
In a previous paper was shown that MB was very active in vitro against laboratory-reared strains from various geographic regions (concentration of MB required to inhibit 50% of the parasite growth IC50: 5 nM) and that late rings and early trophozoites were the most sensitive stages; while early rings, late trophozoites and schizonts were less sensitive (12). Furthermore, combination of MB with commercially available antimalarials resulted in: antagonism with amodiaquine; antagonism/additivity with atovaquone, doxycycline, pyrimethamine; additivity/lack interaction with artemether, chloroquine, mefloquine, primaquine; and synergy with quinine (12). The model described in this study (on BJAB cells) showed MB with CC50 of 577 ± 12 nM (Figure 4) while doxorubicin and CQ, used as controls, had CC50 of 416 ± 8 and 11563 ± 1647 nM respectively. Thus, MB was as toxic against BJAB cells as doxorubicin, and almost 20 times more toxic than CQ. Results were surprising since previous studies reported that MB had CC50s of 1610 and 26700 nM on KB and J111 cells (3, 17). Nevertheless, Kirszberg et al. (18) showed that MB was more toxic on leukaemia cells, than on normal cells. This could explain our results, because BJAB cells are derived from lymphoblast-like lines. A previous study measured the IC50 on P. falciparum chloroquine sensitive (F32, HB3) and resistant strains (FcB1 and FcM29) (12). With the data on BJAB cells found in this study and those previously described, the Selectivity Index (SI) was calculated. SI is defined as the ratio between concentration which produces a cytotoxic effect (CC50) and the antimalarial concentration which causes the desired effects (IC50) (Table 1). The values of SI for CQ were higher than those obtained with MB on sensitive strains (on HB3 the CC50 was 321 times higher than the CI50). On the contrary, MB had highest SI values on resistant strains (Figure 5).
Table 1. General Results.
These results complete those obtained in previous works (12, 13) and confirm the interest of MB as an ancient effective molecule that could be integrated into a new low cost antimalarial therapy, after confirmation of the benefit/risk ratio by further clinical trials.
Figure 1. Methylene blue: 3,7-Bis(dimethylamino)phenothiazin-5-ium chloride; C16H18ClN3S • xH2O; m.w. 319.85 (anhydrous base).
Figure 2. Effective Dose 50 (mg/kg/day) of MB on P. berghei ANKA and P. yoelii nigeriensis rodent malaria.
Figure 3. Effect of MB (100 mg/Kg) on hepatic stage of P. yoelii yoelii infected mice.
Figure 4. Cytotoxic Concentration 50 (CC50) of methylene blue (MB), doxorubicin and chloroquine (CQ) on BJAB cells.

Figure 5. Selectivity Index of MB and CQ on different P. falciparum strains (chloroquine sensitive strains: F32, HB3 chloroquine resistant strains: FcB1, FcM29) in culture.

BIBLIOGRAPHIC REFERENCES
1 Pan American Health Organization (PAHO). Regional Strategic Plan for Malaria in the Americas 2006-2010. Washington (DC): Publications of the PAHO; 2006. [ Links ]
2 Mutabingwa TK. Artemisinin-based combination therapies (ACTs): Best hope for malaria treatment but inaccessible to the needy! Acta Trop 2005;95:305-15. [ Links ]
3 Atamna H, Krugliak M, Shalmiev G, Deharo E, Pescarmona G, Ginsburg H. Mode of antimalarial effect of methylene blue and some of its analogues on Plasmodium falciparum in culture and their inhibition of P. vinckei petteri and P. yoelii nigeriensis in vivo. Biochem Pharmacol 1996;51:693-700. [ Links ]
4 Guttmann P, Ehrlich P. Ueber die Wirkung des Methylenblau bei Malaria. Berlin Klin Wochenschr 1891;28:953-6. [ Links ]
5 Ferreira. Treatement of malaria with methylene blue. Lancet 1893;821-2. [ Links ]
6 Schulemann W. Synthetic anti-malarial preparations. Proc R Soc Med 1932;25:897-905. [ Links ]
7 Wainwright M, Amaral J. The phenothiazinium chromophore and the evolution of antimalarial drugs. Trop Med Int Health 2005;10:501-11. [ Links ]
8 Schirmer RH, Coulibaly B, Stich A, Scheiwein M, Merkle H, Eubel J, et al. Methylene blue as an antimalarial agent. Redox Rep 2003;8:272-5. [ Links ]
9 Wainwright M, Amaral J. The phenothiazinium chromophore and the evolution of antimalarial drugs. Trop Med Int Health 2005;10:501-11. [ Links ]
10 Mandi G, Witte S, Meissner P, Coulibaly B, Mansmann U, Rengelshausen J, et al. Safety of the combination of chloroquine and methylene blue in healthy adult men with G6PD deficiency from rural Burkina Faso. Trop Med Int Health 2005;10:32-8. [ Links ]
11 Meissner PE, Mandi G, Witte S, Coulibaly B, Mansmann U, Rengelshausen J, et al. Safety of the methylene blue plus chloroquine combination in the treatment of uncomplicated falciparum malaria in young children of Burkina Faso. Malar J 2005;4:45-54. [ Links ]
12 Garavito G, Bertani S, Rincon J, Maurel S, Monje MC, Landau I, et al. Blood schizontocidal activity of methylene blue in combination with antimalarials against Plasmodium falciparum. Parasite 2007;14:135-40. [ Links ]
13 Garavito G, Monje MC, Maurel S, Valentin A, Nepveu F, Deharo E. A non-radiolabeled heme–GSH interaction test for the screening of antimalarial compounds. Exp Parasitol 2007;116:311-3. [ Links ]
14 Peters W. Chemotherapy and Drug Resistance in Malaria. London: Academic Press;1970. [ Links ]
15 Peters W, Robinson BL. Handbook of animal models of infection. New York: Academic Press;1999. [ Links ]
16 Landau I, Gautret P. Malaria: Parasite Biology Pathogenesis and Protection. Washington (DC): ASM Press;1998. [ Links ]
17 Vennerstrom JL, Makler MT, Angerhofer CK, Williams JA. Antimalarial dyes revisited: xanthenes, azines, oxazines, and thiazines. Antimicrob Agents Chemother 1995;39:2671-7. [ Links ]
18 Kirszberg C, Rumjanek VM, Capella MA. Methylene blue is more toxic to erythroleukemic cells than to normal peripheral blood mononuclear cells: a possible use in chemotherapy. Cancer Chemother Pharmacol 2005;56:659-65. [ Links ]
ACKNOWLEDGEMENTS
Giovanny Garavito was awarded a PhD fellowship supported by the Programme Alßan, the European Union Programme of High Level Scholarships for Latin America (Scholarship No. E04D039384CO).
Thanks to Landau I. (Museum National d' Histoire Naturelle, France), Ginsburg H. (Hebrew University of Jerusalem, Israel), and Perea-Sasiaín J. (Universidad Nacional de Colombia, Colombia), for these appreciated illuminating discussions. We also wish to thank the members of the Centre National de Référence de la Chimiorésistance du Paludisme (CNRCP) aux Antilles – Guyane of the Institut Pasteur de la Guyane for their technical support.
Recibido: Noviembre 11 de 2007 Aceptado: Marzo 4 de 2008
* Autor a quien se debe dirigir la correspondencia: ggaravitoc@unal.edu.co