Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Vitae
Print version ISSN 0121-4004
Vitae vol.18 no.2 Medellín May/Aug. 2011
FOODS: SCIENCE, TECHNOLOGY AND ENGINEERING
OPTIMIZATION OF THE CROSSFLOW MICROFILTRATION OF ARAZÁ JUICE (Eugenia stipitata) UNDER DIFFERENT OPERATION MODES
OPTIMIZACIÓN DEL PROCESO DE MICROFILTRACIÓN TANGENCIAL DE JUGO DE ARAZÁ (Eugenia stipitata) A DIFERENTES MODOS DE OPERACIÓN
Angelo G. RAMIREZ L.1; Diego F. de los RIOS C.1; Carlos A. VÉLEZ P.1; Heidy L. GALLEGO O.2
1 Escuela de Ingeniería de Alimentos, Universidad del Valle, A.A. 25360 Cali, Colombia. Tel: 057(2) 3212277.
2 Escuela de Ingeniería de Alimentos, Universidad del Valle, A.A. 25360 Cali, Colombia. Tel: 057(2) 3212277. heidy.gallego@correounivalle.edu.co.
Received: 18 January 2011; Accepted: 20 June 2011
ABSTRACT
This study analyzed the effects of temperature and transmembrane pressure on the crossflow microfiltration process of arazá (Eugenia stipitata) juice treated with a commercial pectic enzyme preparation, thus finding the appropriate operation values of the process. Clarified arazá juice was obtained with a crossflow microfiltration pilot plant equipped with ceramic membranes with a 0.48 m2 total effective filtration area and mean pore diameter of 0.2 µm. The juice was evaluated at transmembrane pressures 1.5, 3.0 and 4.5 bar, and at temperatures of 30, 35 and 40ºC at different volumetric reduction factors. The tests were carried out using three systems (total recirculation, concentration, and continuous mode). In total recirculation, it was found that the most influential variable was the transmembrane pressures, and that the partial enzymatic liquefaction of the arazá juice, prior to microfiltration, produced an unusual pattern of permeate flux, characterized by an increase following an abrupt decrease at 4.5 bar and 6.5 m/s. In this case, the highest values of the flux were obtained when compared with those obtained during the crossflow microfiltration in concentration mode. After reaching the value of volumetric reduction factor (3.2), during the crossflow microfiltration in continuous mode, it was not necessary to stop the process as the volumetric reduction factor remained constant for the continuous removal of retained, achieving a high permeate flux in a short period of time 319 L/(h·m2), thus adding to the economic viability of the process.
Keywords: Microfiltration, ceramic membranes, juices, enzymatic liquefaction, volumetric reduction factors.
RESUMEN
En este trabajo se analizaron los efectos de la temperatura y la presión transmembrana sobre el proceso de microfiltración tangencial de jugo de arazá (Eugenia stipitata) tratado con una preparación comercial de enzimas pectolíticas, encontrando los valores adecuados de operación. Se obtuvo un jugo clarificado de arazá con un equipo piloto de microfiltración tangencial provisto de membranas cerámicas de 0,48 m2 de área total efectiva de filtración, diámetro promedio de poro de 0,2 µm, evaluado a diferentes presiones transmembrana de 1,5; 3,0 y 4,5 bar y temperaturas de 30, 35 y 40ºC, a diferentes factores de reducción volumétrica. Las pruebas fueron llevadas a cabo utilizando tres modos de operación: recirculación total, concentración y continuo. En recirculación total, se encontró que la variable más influyente sobre el proceso fue la presión transmembrana y que la licuefacción enzimática parcial realizada al jugo de arazá, previo a la microfiltración, produjo un patrón inusual del flux de permeado, caracterizado por un incremento después de una disminución abrupta cuando se trabajó a 4,5 bar y 6,5 m/s. En este caso se encontraron los valores más altos del flux al compararse con los obtenidos durante la microfiltración tangencial en modo de concentración. Una vez alcanzado el valor de factor de reducción volumétrica (3,2) durante la microfiltración tangencial en modo continuo no fue necesario detener el proceso, ya que el factor de reducción volumétrica se mantuvo constante durante la eliminación continua de retenido, lográndose altos flujos de permeado en corto tiempo (319 L/(h·m2)), facilitando la viabilidad económica del proceso.
Palabras clave: microfiltración, membranas cerámicas, jugos, licuefacción enzimática, factor de reducción volumétrica.
INTRODUCTION
Arazá (Eugenia stipitata Mc. Vaugh) belongs to the Myrtaceae family and is native to the western Peruvian Amazon region. Two species are known: stipitata and sororia, and the latter yields a larger fruit with attractive aromas and flavors. The weight of the sororia subspecies fruits can range between 100 and 350 g (1). This is the most cultivated species due to its high productivity, its pulp yielding (70% approximately), and its resistance to diseases, drought, and high soil aluminum saturation. Araz á trees can be found in the following colombian states: Caquetá, Guaviare, Amazonas, Caldas, Villavicencio, Meta, Cundinamarca, Antioquia, and Putumayo. It is produced continuously throughout the year, with harvests every two months (2).
The arazá pulp contains 90% of water, 0.60 g of protein, 0.20 g of fat, 8.90 g of carbohydrates, 23.3 g of vitamin C, and 0.40 mg of β-carotene (vitamin A). Arazá is suitable for direct consumption and for making jams, juices, juice cocktails, etc. It can be processed with its peel at 20ºC without losing its physical and chemical properties. The best temperature for storage conservation of the fruit is 13ºC, with a relative humidity of 75% (2). At room temperature, arazá is extremely perishable, and at low temperatures it is very sensitive to thermal damage (3, 4). One way to ensure the conservation of the physico-chemical properties and the microbiological stability of the juice obtained from the arazá pulp is through the application of membrane technology, such as the crossflow microfiltration (CFM).
CFM applied to fruit juice is considered to be a treatment of ''cold sterilization,'' thus replacing thermal pasteurization (5). Thanks to the nominal pore size (θ ≤ 0.2 µm), it is possible to separate fine particles in suspension, macro-molecules, bacteria, and viruses while reducing molds, yeasts and plate count, thus ensuring a clear juice (transparent and homogeneous), suitable for consumption (6) with greater characteristics of freshness and overall quality, compared to the characteristics of the fresh juice (7).
CFM has been applied successfully to juices made of fruits from temperate climate, such as apple and grape, which have a relatively low pulp contain. Juices are treated with enzymes prior to filtration in order to hydrolyze soluble polysaccharides that increase viscosity (8). Even though, it can be used nowadays in the industrialization of tropical and exotic fruits sensitive to heat treatment, such as arazá.
Nevertheless, one of the problems in the microfiltration of pulp-rich juices is the formation of a layer of particles on the surface of the membrane, which negatively affects the equipment performance. This is the case of tropical fruits, which require a more aggressive pre-enzyme treatment, not only to reduce viscosity, but also to fractionate the insoluble polysaccharides of the cell wall retained by the membrane (9). This way, a high permeate f lux may be obtained, which also depends on the mode of operation and the established working conditions. During the microfiltration of passion fruit juice, using ceramic membranes with a pore size of 0.2 µm (4), it was found that a total circulation at 36°C, combined with low transmembrane pressure (1.50 bar), a velocity of 7 m/s, and a high enzyme concentration (1 mL3/L) provide the highest f lux (113 L /(h·m2). In a previous study (10), it was observed that the final permeate flux increased 25 to 35% with an enzyme treatment at low velocity (v = 4 m/s), and at high velocity (v = 6 m/s) and high pressure (P = 1.1 bar) during the microfiltration of umbu with a 0.2 µm polypropylene membrane. At high velocity and low pressure (v = 6 m/s, P = 1.1 bar), the enzyme addition did not increase permeate flux. The positive enzyme effect in permeate flux was also observed by (6), who obtained a 100% increase of permeate flux (54 - 109L/h·m2) with the addition of pectinolytic enzymes in a study of acerola juice microfiltration (11). Reported a better flux with an enzyme pretreatment during apple juice microfiltration (an increase of 32%, from 25 to 33 kg/h·m2), and ultrafiltration (an increase of 43%, from 7 to 10 kg/h·m2). In most of the studies which were carried out with filtration membranes, enzymes are added to hydrolyze the pectic substances. In the case of passion fruit juice, a 17% reduction in total solids and a 57% pectin content reduction may be achieved after the enzyme is added (12).
This study analyzed the effect of temperature and transmembrane pressure on the crossf low microfiltration process of arazá juice treated with enzymes in order to find appropriate operation values and determine the industrial feasibility of the process.
MATERIALS AND METHODS
Plant material and sampling
Trials were performed using arazá pulp (Eugenia stipitata ssp sororia), processed at the ''Agropaz'' Cooperative facilities, located in the municipality of Jamundí (Valle del Cauca, Colombia). The pulp (free of seeds and epicarp) was packed in 1 kg polyethylene bags, and stored at -4°C for using it later in the tests, in which a slow freezing process was conducted in an industrial freezer.
Physical parameters evaluated
The soluble and insoluble solids in permeate and retained, respectively, a discontinuous refractometer Abbe (Atago® model 1T, Japan) was used. The viscosity of the samples was determined at 30ºC using a Cannon-Fenske® capillary viscometer type 150 on a 10 mL sample. Suspended insoluble solids (SIS) were determined after centrifuging a 20 g sample for 7 minutes and, then, draining the supernatant. The samples were analyzed in triplicate.
Food processing equipment and control
The partially automated CFM equipment (TIA. Applied Industrial Techniques) consisted of two multichannel profile tubular ceramic membranes (Membralox®, model 1P19-40) with a 0.48 m2 total effective filtration area and a mean pore diameter of 0.2 µm, two pumps (supplying –eccentric piston– and circulation –centrifugal), and a tubular heat exchanger regulated with a proportional controller (PI) featuring an ON/OFF combination and a pulse width modulator (PWM) (figure 1).
The temperature at the outlet of the exchanger was measured using an electronic sensor RTD PT-100 (class B), with a temperature range from 0 to 100°C. The measurement of the pressure at the entrance and exit of the filtration module consisted in two pressure transmitters (PT1 and PT2) piezo resistive from 0.5 to 10 bar and a VP1 proportional valve with proportional integral derivative (PID). Communication signals from pressure transmitters and flow, which are sent to the proportional valve (PV1), were in the standard range (4 to 20 mA). The filtration unit has a 50-L feed tank, which was filled up to 30 L of juice previously hydrolyzed with 0.6 mL/L of a commercial pectic enzyme preparation (Citolase M-102, Gist Brocadest®,Seclin- France) (13).
The process started with the maximum tangential velocity of 6.5 m·s-1 and the maximum operating temperature (40°C). A modified fast start-up process was used (14) to achieve the maximum value of tangential velocity during the first seconds of filtration, and thus slowly getting the value of the transmembrane pressure; this mode of start-up allows decreasing the mass and thickness of the fouling layer, while decreasing the resistance to filtration (15).
The TMP variation (1.5, 3.0 and 4.5 bar) and the temperature variation (30, 35 and 40ºC) at a fixed tangential velocity of 6.5 m·s-1 were performed according to a central composite design, taking into account the ranges allowed during the normal operation of the equipment without denaturalizing the juice. Pressure data in the input and output of membrane module, the tangential velocity, and the operation temperature were recorded and stored, using a high resolution data acquisition system model (PCI 2100 from National Instruments®) (16). Permeate flux was measured using an electromagnetic flow meter (MAGFLO® type MAG 5000, Danfoss). The tests were carried out using three systems as it is described next. Total recirculation and concentration, which consisted in mixing the permeate with the retained material, and then the system was resupplied, keeping the total volume constant. The second system was concentration, in which the permeate was taken off the system and a value for the volumetric reduction factor (VRF) was set, which was defined as the ratio between the feed volume (VF) and the retained volume (VR), according to equation 1. The mass balance was stable during concentration until a certain or infinite period of time.

where:
VRF = Volumetric reduction factor; VP = Permeate volume; VF = Feed volume; VR = Retained volume. The third system was a continuous mode, with which an experiment was conducted with arazá juice (Eugenia stipitata) treated with enzymes, concentrated to a VRF of 3.2. The concentration process was carried out until the level of 27 ºBrix was reached, and soluble solids reached 27% (similar to those of the juice without enzyme treatment). To recover the original membrane permeability (500 L/h·m2), which was reduced during the operation due to pore plugging and subsequent sedimentation, the cleaning procedure recommended by Membralox® was used. This procedure consists in rinsing with water before and after cleaning the membranes with 2% sodium hydroxide solution to reach working temperature, recirculation (50°C/15 min without filtration/15 min with filtration). After rinsing with water at 50ºC, a step with nitric acid 1% at 50°C was sporadically added.
The tuning controllers (P, PI and PID) of the TMP and the temperature was made taking into account the ultimate gain methods and response curves near the pressure levels studied, making fine parameter adjustments for both pressure and temperature, following Ziegler and Nichols methods (17). The pressure tuning was performed at 35°C and at pressures levels of 1.5, 3.0 and 4.5 bar. While the temperature loop tuning was performed on a single pressure level of 3.0 bar at 30, 35 and 40°C. For both cases, the most appropriate driver was a PI.
Statistical analysis
The statistical analysis was made based on the response surface implemented in the Statgraphics® package in order to predict the best operating conditions (temperature and TMP) for obtaining a high permeate flux. Additionally, an analysis of variance (ANOVA) was conducted to obtain a model of the permeate flux as a dependent variable, and TMP and temperature as independent factors.
RESULTS AND DISCUSSION
Optimal crossflow conditions with total recirculation
Effect of the TMP and temperature on the flux
By increasing the temperature and the pressure, the permeate flux increases until it reaches a steady state. In all cases, the TMP of 1.5 bar was too low to achieve high permeate flows over time, independently of the temperature applied (figure 2). This behavior is consistent with those reported by (12), who found that during the ultrafiltration of passion fruit juice (Passiflora edulis), the flux increased with the variation of the temperature between 30 and 40ºC, and with pressure TMP variation between 0.6 and 1.5 bar. The influence of temperature on the permeate f lux may be due to the decreased feed viscosity and the increased diffusion coefficient, which cause an increase in mass transfer and velocity permeate (18, 19).
The partial enzymatic liquefaction of insoluble cell wall polysaccharides of the juice prior to microfiltration produced an unusual flux pattern, characterized by a f lux increase after an abrupt decrease. This increase is not only due to a viscosity decrease but also to an important decrease of the concentration of suspended solids in the retained during microfiltration (figure 3). The soluble solids concentration decreased from 15 to 11% after 100 minutes of microfiltration, this did not happen with the control juice (single enzyme).
A 30ºC temperature was not sufficient to increase the permeate flux (which at the beginning of the process decreased with the applied TMP). Three clearly marked stages were observed: the first one is the accelerated decrease of permeate flux during the first minutes of operation; in the second one, the permeate flux decreases slowly, and then it begins to increase until it stabilizes (third stage). Meanwhile, at 35 and 40ºC there are two stages, corresponding to an increase and stabilization of the permeate flux. The dramatic f lux decrease in the opening minutes of the CFM could be due to the concentration polarization, which results from the concentration of solute in the liquid phase adjacent to the membrane, and from the increased thickness of the layer fouling with the increasing TMP (20).
The influence of temperature on the flux is less significant with the increasing TMP (temperature vs. TMP interaction, which is significant at p < 0.05) (figure 4), while when the pressure is increased, the space between the isotherms is reduced.
Additionally, the interaction indicated that as pressure increases, the slope of the isobar lines are close to zero (figure 5), which suggests that at a pressure of 4.5 bar, temperature did not affect the permeate flux.
The previously described observations agree in some degree with the observations made by (21), who during the microfiltration of a model beer (composed of dextrin and protein) found that by increasing transmembrane pressure and tangential flow velocity, the steady-state f lux was also increased. In the same purport (22), also found that by increasing the TMP, the permeate flux also increases without having a linear relationship between the variables of these two operations. The dramatic decrease of the permeate flux in the first minutes of the CFM (first stage of the process) occurred during the formation of a cake layer. According to (23), this phenomenon is strongly influenced by time and transmembrane pressure. Moreover, who worked with membrane technology for the separation of biodiesel and glycerol (24), corroborated that transmembrane pressure has a great inf luence on the process, showing an improved performance when working with a 0.2 µm membrane and at a pressure of 2 bar; these parameters are similar to those used in this study. Other authors, including (25), have found that a high transmembrane pressure causes the deformation of molecules (as in the case of dextran molecules), which influences the reduction of the fouling layer until reaching a final adsorption equilibrium in the pores of the membrane. On the other hand, claim that the cake formation mechanism controls the membrane plugging after the 20 minutes of operation (26), until achieving a quasi-steady flux state. At a certain time, plugging of the membrane causes an increase in total resistance (Rt), due to an increased dragging force towards the output retained, which is caused by a constant flow velocity (20). This fact translates into a low rejection and a low volume concentration of particles in the cake layer.
In all cases, the repeatability of the permeate flux over time (σn-1 < 5%), evaluated regarding the central point (3 bar and 35°C) and taking TMP and temperature as independent variables, was satisfactory.
The process was modeled by means of a surface response (figure 6), which shows the highest permeate flux values (325.58 and 319.03 L/h·m2 at a pressure of 4.5 bar and at temperatures of 35ºC and 40°C, respectively). On the contrary, the lowest values (231 and 257) L/h·m2 were recorded at 1.5 bar and at temperatures of 30 and 35ºC, confirming that TMP is the most influential variable.
It can also be noticed that a higher permeate flux was obtained at a TMP of 4.5 bar and 40°C. However, due to the minor influence of temperature on the flux when increasing TMP, working conditions of 35ºC and 4.5 bar TMP are the final recommendation for well-known better preservation of the nutritional and sensory properties of juice.
The comparison of surface flux response regression vs. T and TMP was useful for obtaining the data fit model (equation 2) with a standard deviation (σ) of 3.28 and a coefficient of variation (R2) of 99.6%.
Permeate

The interaction of the ANOVA model mean factors, taking temperature and TMP as factors, were significant at p < 0.05.
Experiments with concentration
Effect of the volumetric reduction factor (VRF) on permeate flux
The values for permeate flux and VRF were much lower than those obtained with the total circulation method it the test, due to the additional resistance created by the accumulation of particles on the surface of the membrane (figure 7).
Permeate flux decreased during the first 30 minutes of microfiltration, remained constant at 220 L/h-1·m-2 from the VRF of 2 to 3.2, and then it abruptly decliney. The reason for this behavior is that the concentration of solids causes the decrease of permeate flux from the beginning of the filtration and, consequently, there is an inverse relationship between VRF and the volume retained, as it is shown in the crossflow microfiltration process of different tropical fruit juices (7, 8).
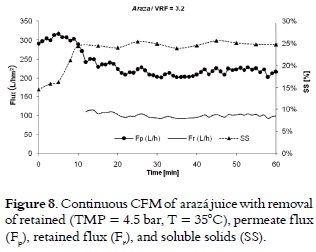
Continuous CFM
There was no significant decrease in the permeate flux (Fp), which was 220 ± 5 L/h·m2, presenting a 69% yield (figure 8). Similar results were obtained with different tropical fruit juices (7).
As soon as the desired VRF is reached, it is not necessary to stop the process, since the VRF can be kept constant with a continuous removal withheld. In addition, this process significantly reduces the residence time of the retained, resulting in a better quality of it.
CONCLUSIONS
In all the cases studied, the permeate f lux increased with the increase of temperature and TMP. However, the graphs for the interaction of temperature vs TMP showed that the influence of temperature becomes less significant with the in crease in TMP.
Based on the obtained permeate flux values, when working in the total recirculation mode and due to the limited influence of temperature at a high TMP, it was established that the best working conditions were 4.5 bar and 35°C, which guarantee the conservation of the arazá juice organoleptic properties.
The microfiltration of arazá juice (Eugenia stipitata) with a 3.2 VRF in a continuous operation had a yield of 69%, maintaining the SS content stable and permeate flux at 4.5 bar and at 35°C. Therefore, the continuous operation could be used in an industrial plant clarified arazá juice.
ACKNOWLEDGMENTS
The authors would like to thank Colciencias (Colombia) and Universidad del Valle for their financial support to carry out this study. The authors would also like to thank Universidad del Valle's Professor Argemiro Arboleda A., who translated this article from Spanish into English.
REFERENCES
1. Mantilla Cardenas LM, Piñeres Vergara R, Hernandez MS. Bases técnicas para el aprovechamiento agroindustrial de especies nativas de la amazonia [Internet]. Colombia: Instituto amazónico de investigaciones científicas – SINCHI; 2004 Apr. Disponible en: http://www.fao.org/fileadmin/templatyes/inpho/documents/ad418s00.pdf. [ Links ]
2. Quevedo García E. Aspectos agronómicos sobre el cultivo del arazá (Eugenia stipitata Mc Vaugh). Agronomia colombiana. 1995; 12 (1): 27-65. [ Links ]
3. Carrillo MP, Hernandez MS, Barrera JÁ, Martinez O, Fernandez- Trujillo JP. 1-methylcyclopropene delays arazá ripening and improves post-harvest fruit quality. LWT-Food Sci Technol. 2011 Jan; 44 (1): 250-255. [ Links ]
4. Hernandez MS, Martinez O, Fernandez-Trujillo JP. Behavior of arazá fruit quality traits during growth, development and ripening. Scientia Hort. 2007 Feb 5; 111 (3): 220-227. [ Links ]
5. Carneiro L, dos Santos Sa I, dos Santos Gomes F, Matta VM, Correa Cabral LM. Cold sterilization and clarification of pineapple juice by tangential microfiltration. DesalinatioN. 2002 Sept 10; 148 (1-3): 93-98. [ Links ]
6. Matta VM, Moretti RH, Correa Cabral LM. Microfiltration and reverse osmosis for clarification and concentration of acerola juice. J Food Eng. 2004 Feb; 61 (3): 477-482. [ Links ]
7. Vaillant F, Millan A, Dornier M, Decloux M, Reynes M. Strategy for economical optimization of the clarification of pulpy fruit juices using crossf low microfiltration. J Food Eng. 2001 Apr; 48 (1): 83-90. [ Links ]
8. Vaillant F, Millan P, O'Biren G, Dornier M, Decloux M, Reynes M. Crossf low microfiltration of passion fruit juice after partial enzymatic liquefaction. J Food Eng. 1999 Dec; 42 (4): 215-224. [ Links ]
9. Vaillant F, Perez AM, Viquez F. Microfiltración de flujo tangencial: una alternativa para el procesamiento de frutas tropicales. La Alimentación Latinoamericana. 2004; 252: 38-46. [ Links ]
10. Ushikubo FY, Watanabe AP, Viotto LA. Microfiltration of umbu (Spondias tuberosa Arr. Cam.) juice. J Membrane Sci. 2007 Feb 1; 288 (1-2): 61-66. [ Links ]
11. Yu J, Lencki RW. Effect of enzyme treatments on the fouling behavior of apple juice during microfiltration. J Food Eng. 2004 Aug; 63 (4): 413-423. [ Links ]
12. Jiraratananon R, Chanachai A. A study fouling in the ultrafiltration of passion fruit juice. J Membrane Sci. 1996 Mar 6; 111 (1): 39-48. [ Links ]
13. Ayala DF, Argumedo FR. Aplicación de tecnologías de membranas para la clarificación de jugo de arazá [Trabajo de grado de Ingeniería Química]. [Cali, Colombia]: Universidad del Valle: 2004. 55 p. [ Links ]
14. Dornier M, Petermann R, Decloux M. Influence of start-up procedure on crossflow microfiltration of raw cane sugar. J Food Eng. 1995; 24 (2): 213-224. [ Links ]
15. Choi H, Zhang K, Dionysiou DD, Oerther DB, Sorial GA. Influence of cross-flow velocity on membrane performance during filtration of biological suspension. J Membrane Sci. 2005 Feb 15; 248 (1-2): 189-199. [ Links ]
16. Franco E, Ramos C, Maya AM. Aplicaciones de Linux para simulaci ón y control de procesos–software GNU y Scicos. Revista Energía y computación 2nd edition. 2005; 11 (20): 54-59. [ Links ]
17. Smith CA, Corripio B. Control automatico de procesos. México: Limusa; 1991. [ Links ]
18. Cassano A, Drioli E, Galaverna G, Marchelli R, Di Silvestro G, Cagnasso P. Clarification and concentration of citrus and carrot juices by integrated membrane processes. J Food Eng. 2003 Apr; 57 (2): 153-163. [ Links ]
19. Cassano A, Jiao B, Drioli E. Production of concentrated kiwi fruit juice by integrated membrane process. Food Res Int. 2004 Mar 1; 37 (2): 139-148. [ Links ]
20. Vyas HK, Bennett RJ, Marshall AD. Performance of crossflow microfiltration during constant transmembrane pressure and constant f lux operations. Int Dairy J. 2002 Jan 1; 12 (5): 473-479. [ Links ]
21. Thomassen JK, Faraday DBF, Underwood BO, Cleaver JAS. The effect of varying transmembrane pressure and crossflow velocity on the microfiltration fouling of a model beer. Sep Purif Technol. 2005 Jan; 41 (1): 91-100. [ Links ]
22. Tung KL, Li YL, Wang S, Nanda D, Hu ChCh, Li ChL, et al. Performance and effects of polymeric membranes on the deadend microfiltration of protein solution during filtration cycles. J Membrane Sci. 2010 Apr 15; 352 (1-2): 143-152. [ Links ]
23. Vaillant F, Pérez AM, Acosta O, Dornier M. Turbidity of pulpy fruit juice: A key factor for predicting cross-flow microfiltration performance. J Membrane Sci. 2008 Nov 15; 325 (1): 404-412. [ Links ]
24. Sérgi Gomes MC, Curvelo Pereira N, Davantel de Barros ST. Separation of biodiesel and glycerol using ceramic membranes. J Membrane Sci. 2010 Apr 15; 352 (1-2): 271-276. [ Links ]
25. Hwang KJ, Sz PY. Filtration characteristics and membrane fouling in cross-f low microfiltration of BSA/dextran binary suspension. J Membrane Sci. 2010 Feb 1; 347 (1-2): 75-82. [ Links ]
26. Yazdanshenas M, Soltanieh M, Tabatabei-Nejad SAR, Fillaudeau L. Cross-flow microfiltration of rough non-alcoholic beer and diluted malt extract with tubular ceramic membranes: Investigation of fouling mechanisms. J Membrane Sci. 2010 Oct 15; 362 (1-2): 306-316. [ Links ]