INTRODUCTION
Despite all the preventive steps taken throughout the world, the COVID-19 pandemic has significantly impacted human life. It is highly urged that only an effective and safer vaccination can prevent this epidemic. With the emergence of COVID-19 in China, vaccine development initiatives were launched, first in China and then internationally, after the World Health Organization proclaimed the disease a pandemic. Several technologies have been created to generate the safest and most potent vaccines, including Inactivated vaccines, Live attenuated vaccines, RNA and DNA vaccines, Viral vectors, and Protein subunits. SARS-CoV-2 is a positive-strand RNA virus related to coronaviruses that cause acute respiratory distress syndrome called Severe Acute Respiratory Syndrome (SARS) (1). Since the COVID-19 outbreak in China in 2019, this virus has been responsible for the death of about 5,978,096 people around the world reported by (World Health Organization) WHO (Feb 26, 2022) (2). The COVID-19 epidemic has wreaked havoc on social, psychological, and economic systems worldwide. Complete programs like lockdowns reverse transcription-polymerase chain reaction (RT- PCR) testing, isolation, lockdowns, social distance, and sanitation has been implemented (3). Because vaccines are the only effectual mode of control for the COVID-19 pandemic, the nonstop spread of the virus brought together by global pharmaceutical companies, research institutions have to initiate the development of vaccines as soon as possible (4). Currently, several vaccinations have received worldwide authorization for emergency usage. However, vaccine acceptance is necessary for human survival that all people should be vaccinated as soon as possible. The difficulties in the technology transfer of vaccines from Research and Development (R&D) to the manufacturing site were described in this review (5,6).
VACCINE FORMULATIONS
Inactivated Vaccines
Viruses, bacteria, and other pathogenic microorganisms that have been developed in culture and subsequently destroyed are the components of an inactivated vaccine. Inactivated vaccine pathogens are created under controlled conditions and destroyed to reduce their infectiousness and prevent vaccination-related illness. Inactivated vaccines were developed in the late 1800s and early 1900s to treat diseases like cholera, plague, and typhoid. Numerous diseases can now be vaccinated using inactive vaccines, including influenza, polio, rabies, hepatitis A, and pertussis. Immunologic adjuvants and multiple booster injections may be essential for some immunizations to initiate an immune response against the pathogen. Inactivated pathogens evoke a weaker response than live infections (7).
Some of the Inactivated vaccines developed for COVID-19 are, the Chinese company Sinovac had created an inactivated vaccine called CoronaVac (8). Beijing Institute of Biological Products collaborates with the Chinese Center for Disease Control and Prevention on developing the BBIBP-CorV vaccine, an inactivated vaccine. Covaxin was developed in partnership with the National Institute of Virology (NIV) in Pune, the Indian Council of Medical Research (ICMR) in New Delhi, and the Indian company Bharat Biotech International Limited (Hyderabad). Kazakhstan’s Research Institute for Biological Safety Problems (QazCOVID) has been developing an inactivated vaccine. AstraZeneca and Shenzhen Kangtai Biological Products collaborated to develop the inactivated vaccine (9). Two inactivated vaccines developed by Iran are COVIran Barekat from Shifa Pharmed Industrial Company and FAKHRAVAC (MIVAC) from the Organization of Defensive Innovation and Research.
Live Attenuated Vaccine
Virulence of a disease can be reduced while still retaining the vaccine’s viability through attenuated vaccines. Attenuation is altering an infectious agent so that it is no longer dangerous or harmful. Vaccines that are attenuated provide a robust immune response that lasts for a long time (10). The attenuated vaccine induces a more robust and long- lasting immune response with a rapid protection onset than inactivated vaccines. One of the first and most effective immunization strategies was the use of live, attenuated vaccines. British doctor Edward Jenner utilized cowpox to immunize youngsters against smallpox in the 18th century; thus smallpox was successfully eradicated. Measles, polio (sabin vaccine), rotavirus, smallpox, TB, varicella-zoster (chickenpox), and yellow fever are all examples of successful live attenuated vaccinations (11). CD8+ and CD4+ T lymphocytes and other pathogen-specific cells are produced as a result of these Vaccines. Cells and molecules can prevent or decrease infection by killing or producing interleukins from infected cells or other molecular entities. Some vaccines may cause more or less specific effectors to be released. Immune responses that are mediated by CD8+ cytotoxic T cells and T-dependent antibodies are facilitated by live attenuated vaccines. The immune system must maintain a sufficient number of these cells for a vaccination to be effective (12). Some examples of live attenuated vaccines are COVAXIN from the Serum Institute of India (India) and Meissa Vaccines (United States of America) for SARS-CoV-2 infection (13).
RNA vaccines
The mRNA- based COVID-19 vaccines were administered to millions of people globally in recent years. Robert Malone experimented in late 1987 to make a molecular stew; he combined messenger RNA (mRNA) with fat droplets. The mRNA was taken up by human cells soaked in this genetic stew, and proteins were produced from it, through this “treating RNA as a medication” was possible. Fatty droplets were utilized for the first time to help mRNA enter a living creature (14,15). In these vaccines the protein molecules trigger an adaptive immune response, instructing the body to identify and remove the infection or cancer cell linked with it (16). The RNA and lipid nanoparticles used in the mRNA formulation help preserve and aid in the absorption of the RNA strands into the cells. Vaccines stimulate the body’s adaptive immune system to manufacture antibodies in response to a given disease. The pathogen’s antigens are targeted by antibodies (17,18).
Some vaccines developed under R&D technology collaboration are the Moderna vaccine (mRNA-1273), developed rapidly in Cambridge, and Massachusetts, with funding from the National Institute of Allergy and Infectious Diseases (NIAID). The UK government has authorized the use of an RNA-based vaccine (BNT162b1) in a clinical study in Denmark and Germany, collaborating with the Danish Ministry of the Interior and Health and Pfizer/BioNTech (Germany). CureVac (Germany) is partnering with Elon Musk (owner of Tesla) on the establishment of “mRNA-factories” capable of being shipped abroad to create billions of immunization doses (19). Arcturus/ Duke-Nus (United States of America/Singapore) is involved in Arcturus (ARCT-021), an RNA vaccine studied in Singapore and the United States (20).
DNA Vaccine
Researchers became interested in genetically engineered vaccines in the early 1990s when plasmid DNA was administered subcutaneously or intramuscularly to produce antibodies against viral and nonviral antigens. Without the need for a reproducing pathogen, DNA vaccines have the potential to produce widespread immune responses, similar to those induced by live-attenuated viral vaccines. Small animal studies have shown promising results for DNA vaccines. Despite their safety and tolerability, their immunogenicity was modest, and proven that DNA vaccines are safe and effective at low doses. Second-generation DNA vaccines increase cellular immune responses in all groups of animal models (21). Additionally, research in bigger animal models demonstrates that newer DNA vaccines may activate CD81 cytotoxic T lymphocytes (CTL) more extensively than previous DNA methods. When it comes to vaccine production and design, DNA vaccines are the most inflammatory manifestations. Antigen-coding genes are introduced into a bacterial plasmid in DNA vaccinations. Genes for mammalian expression and a gene for the spike protein are commonly found in these plasmids (22). Humoral, cellular, and immunological responses are elicited in response to the DNA vaccine. Therefore, electroporation and bio-injection as delivery methods for DNA vaccines are necessary (23).
Examples of DNA vaccines are Cadila Healthcare (ZyCoV-D), and Osaka University/AnGes (Japan) in collaboration with Takara Bio (AG0303-COVID-19) (24); Inovio Pharmaceuticals (United States of America) developed the Inovio vaccine (INO-4800) (25); the Genexine (GX-19) vaccine, created in collaboration with Genexine, Binex, GenNBio, the International Vaccine Institute, Korea’s Advanced Institute of Science and Technology, and Pohang University’s Science and Technology Department (26,27).
Viral vector vaccine
In 1972, genetic engineering was used by Jackson et al. to create recombinant DNA from the SV40 virus. Next, in 1982, Moss and colleagues demonstrated the vaccinia virus as an expression vector. The most commonly employed vectors can produce immune responses, specifically CTLs, by adenovirus and vaccinia virus. Immunogenicity is generally achieved without the use of adjuvants in viral vectors. Interferons and inflammatory cytokines are produced due to the innate immune response triggered by viral components (28,29). It is possible to provide an antigen-specific immunization to an individual using a virus-borne vector. Viral vector vaccines modify a virus to transport a nucleic acid coding for an antigen from an infectious agent to a cell. When a virus is used to deliver a vaccine, it does not infect itself or its antigen source. It does not incorporate the genetic material it provides into a person’s DNA (30,31). Viral vectors come in various forms, including vaccine-vectoring poxviruses, adenoviruses, adeno-associated viruses, retroviruses, lentiviruses, cytomegaloviruses, and Sendai viruses.
Some examples of viral vector vaccines used in corons are the AstraZeneca COVID-19 (AZD1222) vaccine developed by the University of Oxford in the United Kingdom. Gam-COVID-Vac (Sputnik V) vaccine, produced in Russia by the Gamaleya Research Institute of Epidemiology and Microbiology. Cansino Biologics worked with the Academy of Military Medical Sciences China to produce a stabilized form of the SARS-CoV-2 spike protein and vaccine (Convidecia) using a human adenovirus, Ad26, the viral vector (32). GRAd-COV2 is a viral vector vaccine based on Adenovirus created by the Italian company ReiThera. Israel Institute for Biological Research (Israel) - Iibr-100, a viral vector vaccine, and the Serum Institute of India (India) - Covishield, a viral vector vaccine designed and developed by Serum Institute of India (33). Immunity Bio and Nantkwest developed ImmunityBio (hAd5) viral vector vaccine. Vaccines using viral vectors include Vaxart (United States of America), and adenovirus (Ad5) vaccines designed to deliver both spike protein and nucleocapsid DNA to induce cellular and antibody immunity (34,35).
Protein Subunit vaccine
In Protein subunit vaccines, protein complexes consist of single protein molecules combined with other protein molecules. These vaccines contain purified protein species of virus which will boost immunity. The virus genetic code is inserted into another cell (bacteria or yeast) to develop these vaccines; then, the cell will produce virus proteins. These proteins are extracted, purified, and used as active ingredients in the vaccines. In some vaccines, adjuvants are added for stronger and longer-lasting immune responses (36).
Some examples of Protein Subunit Vaccines are Novavax (United States of America) produced the SARS- CoV-2 spike protein antigen using nanoparticle technology for the Novax-CoV2373 vaccine (37). ZF2001 vaccine was developed together by the Institute of Microbiology, the Chinese Academy of Sciences, and Anhui Zhifei Longcom Biopharmaceutical (China) (38). Sanofi Pasteur/ GlaxoSmithKline is developing the VAT00002 vaccine against SARS- CoV-2. Vector Vaccine (Russia) - The Russian Biological Research Center in Russia designed the EpiVacCorona vaccine. Abdala is developed by the Cuban Center for Genetic Engineering and Biotechnology (Cuba). In Australia, Clover Biopharmaceuticals (SCB2019) produced a protein vaccine based on the SARS-CoV-2 s-trimer strain (39). An RBDBV gene-encoding protein subunit vaccination is used at the West China Hospital of Sichuan University (China). In Taiwan, Medigen produces a protein subunit vaccination called Medigen COVID-19. A plant-based vaccine, Kentuchy Bioprocessing (KB-201), was developed utilizing genetically altered Nicotiana benthamiana to make viral proteins comparable to the Medicago (plant- based vaccine) (40). India’s Biological E (BECOV2A) is a protein subunit vaccine. Cov-Pars is a protein subunit vaccine that contains coronavirus-like spike proteins from the Razi Vaccine and Serum Research Institute (Iran). It is given to the patient in a three-part regimen: two injections and one spray on the nose. Moreover, it is the first SARS-CoV-2 vaccine that can be injected and inhaled simultaneously. In the United States, scientists at Walter Reed Army Medical Research Institute have developed a vaccine that targets protein subunits (SpFN). This vaccine Spike Ferritin Nanoparticle is a liposomal formulation (41).
These are the different types of vaccine technologies available globally are presented in Figure 1 and the number of vaccines that got approval and are in current use is shown in Table 1 (42-44).
Table 1 Current status of corona vaccine globally
| Company or Institute | Vaccine | Formulation | Country |
|---|---|---|---|
| Novavax | NVX - CoV 2373 | Protein subunit | USA |
| Chinese Academy of Science, Anhui Zhifei Longcom biopharmaceuticals, and Institute of microbiology | ZF 2001 | Protein subunit | China |
| Sanofi Pasteur/ GlaxoSmithKline | VAT00002 | Protein subunit | France |
| Instituto Finlay de vacunas | Finlay - FR-1 | Protein subunit | Cuba |
| Vector vaccine | Epi vac corona | Protein subunit | Russia |
| Biotechnology of Cuba and the center for genetic engineering | Abdala | Protein subunit | Cuba |
| Medicago | Plant-based VLP | Protein subunit | Canada |
| Oxford University and AstraZeneca | AZD1222 | Viral vector | UK |
| Cansino biologics INC | AD5 - nCoV | Viral vector | China |
| Gamaleya research institute | Sputnik | Viral vector | Russia |
| Janssen/ jhonson & jhonson | Ad26. CoV.S | Viral vector | USA |
| Moderna | mRNA - 1273 | RNA | USA |
| Pfizer/ BioNTech | BNT 162b1 | RNA | UK |
| CureVac | CVnCoV | RNA | Germany |
| Zydus Cadila | ZyCoV - D | DNA | India |
| Beijing Institute of biological products | BBIBP - Corv | Inactivated | China |
| Wuhan Institute of biological products | Inactivated Vero cells | Inactivated | China |
| Sinovac | CoronaVac | Inactivated | China |
| Bharat biotech international limited | Covaxin | Inactivated | India |
| Chinese Academy of medical science | Inactivated Vero cells | Inactivated | China |
| Research institute for biological safety problems | QazCOVID | Inactivated | Kazakhstan |
TECHNOLOGY TRANSFER
Technology transfer refers to steps required for a successful transfer of procedures to achieve full- scale production for marketing, initiated from drug discovery, product development, clinical trials, and finally, commercialization. The knowledge gained thus is helpful to know about the process involved in manufacturing, control strategy approaches for process validation, and ongoing continual improvements. The transfer of process and product knowledge gained between the R&D and production unit is the technology transfer’s primary objective, which can happen among or between manufacturing facilities to achieve product realization. Technology transfer usually involves carrying technology to a group of targets that do not show any special technical skills and cannot create the tool themselves. The technology transfer involves gaining a license for intellectual property rights and extending the property rights and technical expertise to develop firms (45).
Objectives of technology transfer documents
In practice, the technology transfer document provides guidelines and offers general recommendations on the activities required for effective intra or inter-site technology transfer. The aim is to inform thefundamental requirements for an effective transfer to satisfy the regulatory authority set up for the process transfer (46). The guidelines prescribed shall refer to the production of active pharmaceutical ingredients, the manufacture, and packaging of bulk materials, and the manufacture and packaging of finished pharmaceutical products. The criteria set out apply to all forms of dosage, but need to be modified on a case-by-case basis (47).
Technology transfer Protocol
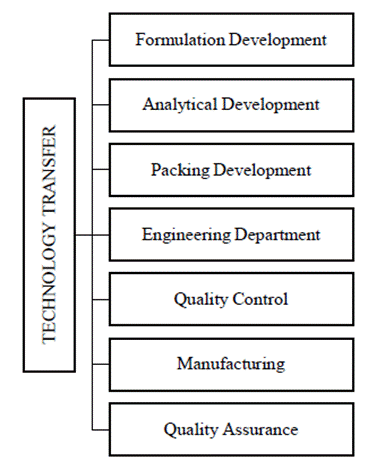
The protocol includes all the precautions to be taken between the sending and receiving sites. The sending site should specify any process robustness issues to the receiving site. Both the sites will collaborate to create a method for transferring relevant process data from the sending to the receiving and implementing a similar procedure in the receiving site. The major areas are Analytical and Production technology transfer. The Technology Transfer Dossier (TTD) is evaluated by the Head of Production, Quality Control, Engineering, and accepted by Quality Assurance. It includes a Process flow map, raw material, and packaging content requirements, in-process and finished product specifications, master model sheet, safety precautions, critical process phases, calculated reaction measurements, process validation procedure, process validation report, reliability data, a variance will be carried out. After the effective transfer of technology, the production unit’s function is to produce a specific product (manufacturing process). If an issue arises, Quality Assurance (QA) will investigate and report back to R&D via Inter-Office Communication (IOC). If appropriate, any deviation in the process must be endorsed on the deviation/change control form (48); various departments involved in technology transfer were shown in Figure 2.
Checklist
A checklist that lists the details should be gathered during the process. I t i n c l u d e s Master Planning Model, Material Manufacturing Guidelines, AnalyticProcesses, Previous Process Validation, Previous Process Analytical Validation, Cleaning Guidelines and Previous Processes, Stability Documents, Excipient Descriptions and Origin, Active Requirements and Source, Main Packaging Materials Specifications and Source, Packaging Orders, Consumer Statements, Product Deviations Log, Analytical Deviations Log, Reject and Rework Register, Sample Test Report, Experiment Boxes, Labels, Leaflets (49).
Risk Management in Technology transfer
Several unpredictable hazards are connected with technology transfer; thus, it is critical to consider and implement a QRM plan to complete a successful technology transfer. It is a continuous, systematic process that assesses, mitigates, and optimizes risks to product quality throughout its life cycle. QRM is a set of science-based, practical judgments resulting from quality management systems. Quality system - investigation, auditing, inspection, documentation, training, and so on are all examples of quality systems (50,51) the flow diagram for risk assessment was presented in (Figure 3).
Importance of Technology transfer
Technology transfer and Collaboration are significant in the battle against COVID-19 (52). The appeal to collaborate has been endorsed by several multinational organizations (53), including the unprecedented global sharing (54) of COVID-19 viral genetic sequences (55), and efforts to share knowledge, intellectual property, and technology, among others (56). There is a lot of collaboration, but also a lot of competition, in the race to make vaccines for COVID-19 safe and effective. Public- private partnerships (PPPs) are often comprised of profit and non-profit organizations that collaborate and benefit from one another. Additionally, they worry about social value and improved health (57). They have played a critical part in the COVID-19 situation (58). PPPs have previously been utilized to aid in vaccine discovery and manufacture and assist impoverished nations in obtaining crucial supplies (59). While the conditions, goals, and financial incentives associated with a global pandemic are distinct from those associated with rare diseases (60). The technology transfer involves sharing knowledge and technical skills and giving away materials, technical infrastructure, and intellectual property rights. In the first case, the two partners work together to build technology and learn more about each other’s strengths and weaknesses. This way, both partners will be better prepared for the future. One partner takes the first steps of innovation, then gives the materials, tools, and intellectual property to another partner, who can take the following steps. This is called “serial innovation.” The most common collaborations between academics and businesses follow this pattern: academic partners usually help with early clinical development, and private businesses help with later stages of development (61). We reviewed the different collaborations that participated in the development of COVID-19 vaccines and the characteristics of these relationships. Our findings suggest that policymakers can strengthen the collaboration by sharing the knowledge, fundamental insights development, and production technology (62).
When the pharmaceutical company AstraZeneca teamed up with Oxford University, they were able to share materials. Both Oxford and its spin-off business Vaccitech have developed a novel platform for vaccine development. This is an example of the collaborative serial invention (63-65). When three businesses collaborated, they pooled their expertise. BioNTech, Fosun Pharmaceuticals, and Pfizer collaborated to share their knowledge (66-68).
CHALLENGES IN TECHNOLOGY TRANSFER
The technology transfer of vaccines includes several challenges from development to marketing. Some of the challenges we collected through the literature survey are discussed below.
Optimal vaccination
Vaccine development is a lengthy and costly process, with several obstacles encountered throughout discovery, and manufacture. The developed vaccine should have some specific characteristics it should be safe and very successful in inducing immunity, should maintain immunogenicity in the presence of poor storage conditions, should be economical, should be toxicologically inert and thermally stable (69).
Time
Developing the vaccine for SARS- CoV2 is a lengthy process that starts from finding the virus to developing and testing the vaccine. Before the pandemic, this was not the case. Before any new vaccine can be sold on the market and widely available in the public domain, it must first be tested for safety and effectiveness, which usually takes at least five years. Due to the rapid increase of COVID-19 cases worldwide, vaccine regulatory agencies at both the international and national levels must accelerate every stage of development to address the world’s urgent vaccination requirement. The scientific community is adopting numerous initiatives to accelerate development, including overlapping clinical stages and modern computer- aided and biotechnological techniques.
Toxicology and unfavorable consequences
The rapid development of vaccinations raises the possibility of adverse effects. At this quick pace of development, there is a strong probability that critical data may be lost or overlooked. Public health may be risked if significant research is not made public. Preclinical research on animal models is often required to ensure that novel vaccination candidates or combination vaccines are safe and readily available before being tested on people. To ensure the conduct of the experiments, they must adhere to good laboratory practice norms and any national animal experimentation legislation. These tests are also critical for determining the vaccine candidates’ physical, chemical, and biological features. While some research organizations have opted out for doing these critical animal investigations amid a global health crisis, others conduct preclinical and first-in-human experiments concurrently. It is a significant change from the way things usually work, and it’s a big problem that needs to be solved quickly. The animal studies may not be 100% accurate in toxicity studies. The symptoms of SARS- CoV-2 can also be seen in animal models, like ferrets, Syrian hamsters, and rhesus macaques, when they are exposed to the virus (70). Thus, even if there is proof that a vaccine can be both safe and effective, very few vaccines have been approved for use by a large group of people. DNA and RNA are examples of new technologies that should be given more attention. Protein and non-protein impurities found in vaccines have caused severe allergic reactions and other problems in the past. Before vaccines can be given out, they must be thoroughly checked for quality during the manufacturing process.
Prolonged protection
Ideally, immunization should provide long-term protection. However, immunization-induced resistance decreases with time, and the extent to which protection is lost varies according to the kind of illness (71). The majority of SARS-CoV2 vaccinations now in clinical studies must be administered twice. It’s too soon to say that any of it will protect you for a long time. Reinfection is another significant factor in how long the protection lasts. A recent study has shown that the virus has returned with genomic evidence. SARS-CoV-2 could spread through the human population even if people could not get it because they were infected or had been immunized.
Additional monitoring of patients who relapse from SARS-CoV2 will help improve the design of a vaccine against the virus (72).
Mutations
During the early phases of the epidemic, there was considerable uncertainty regarding SARS-CoV-2 mutations. Recent investigations, however, have shown that there is no cause to be concerned. It was discovered that all of the glycoproteins from the various strains of SARS-CoV-2 isolated from different countries were quite similar. It indicates that an antibody raised against a particular strain of SARS-CoV-2 would be effective against strains from other nations. However, it is still essential to watch the virus’s genome because we know how quickly viruses change (73).
Adverse Drug Events
The adverse drug reaction (ADE) of a disease is a significant obstacle to the vaccines’ development and therapies since it has the potential to worsen the infection or result in hazardous immunopathology (74,75). There is no evidence that COVID-19 individuals suffer from ADE in vitro, in vivo, or clinically. On the other hand, ADE may have significant effects throughout the disease (76). Thus, it is critical to monitor ADE for a lengthy period following vaccination, particularly during a mass vaccination campaign, since ADE manifests itself after many individuals have been immunized.
Production Price
The vaccine production employs novel technology. The production will be required to adhere to guidelines while developing the vaccine candidate. There will be a cost associated with establishing new facilities and equipment for vaccine manufacturing that adhere to all applicable quality requirements. Additionally, at present there is a worldwide rush to develop a vaccine, there is a risk that this critical compliance stage may get insufficient attention, which might be disastrous. The goal is to vaccinate everyone on the planet. Experts are worried that this might be hard to do because there are not enough resources.
CONCLUSION
In this review, we have discussed the current formulation parameters of COVID vaccines along with how technology transfer can bridge gaps in formulation difficulties. Among all the vaccines formulated, researchers have noted that vaccines prepared using viral vectors pose more risk in formulation and administration than other types. We have also discussed how technology transfer can help in the rapid production of vaccines in this critical hour of need; unlike normal conditions, technology transfer has to adopt new methods to ensure quick and effective technology transfer. Finally, in this review, we have also highlighted the risk assessments involved in the formulation sector and technology transfer that might affect the rapid mass production of COVID vaccines.