Introduction
Tomato (Lycopersicon esculentum) is a plant of the Solanaceae family native to the low Andes. The plants can be propagated by seed or clonally by tip or shoot cuttings, have a high yield of fruit, and have a reasonable biomass and protein content1. Early observational studies linked tomato consumption to reduced prostate cancer risk, which prompted people to use this fruit to prevent this and other conditions such as colorectal and stomach cancer, diabetes, and osteoarthritis. However, although tomatoes as a non-starchy vegetable may decrease the overall risk of cancer and all-cause mortality2, there is no solid scientific evidence to support these uses to date. In contrast, tomato products potentially have beneficial effects in preventing and treating cardiovascular diseases, mainly due to their high content of potent antioxidants, such as vitamin A, carotenoids, vitamin E, and phenolic compounds2-4. A recent systematic review and meta-analysis concluded that standardized tomato extract significantly decreases systolic blood pressure compared to placebo in healthy and hypertensive patients5. Additional studies suggest that in food and therapeutic doses, tomatoes effectively prevent preeclampsia and hyperlipidemia in pregnancy and reduce platelet aggregation6,7.
The global rise of antibiotic resistance has also increased the research interest in the antimicrobial potential of natural products in recent years, including tomatoes. Several studies testing various extracts from different parts of the tomato plant have identified antimicrobial properties against bacteria -particularly Gram-positive-4,8-10, fungi11, and protozoan parasites11. Some Latin American rural populations have used tomato leaf preparations for anti-inflammatory, analgesic, antibiotic, and antiseptic purposes12. In Colombia, for example, tomato leaves as an antiseptic for external use are an accepted medicinal herb13, and mouthwashes based on tomato leaf extracts are commercially available. However, research on the potential activity of tomato leaf extracts against oral microorganisms and in managing oropharyngeal infections is scarce.
S. mutans and P. gingivalis are the primary agents in the pathogenesis and progression of dental caries and periodontal disease, respectively. It is estimated that oral diseases affect nearly 3.5 billion people worldwide, with caries of permanent teeth and periodontal disease being the most common conditions. Globally, around 2 billion people suffer from caries of permanent teeth, and 520 million children suffer from caries of primary teeth14. Severe periodontal diseases affect approximately 14% of the adult population, representing more than one billion cases worldwide14. These oral diseases are largely preventable through lifestyle modification, such as promoting a well-balanced diet low in free sugars and high in fruit and vegetables, improving oral hygiene, and stopping the use of all forms of tobacco. Also, fluoride exposure can substantially reduce the risk of dental caries. Unfortunately, however, in most low- and middle-income countries, the prevalence of these conditions continues to increase15. This public health problem, together with the rapid emergence of antibiotic-resistant bacteria, makes it imperative to search for inexpensive natural products that might aid in controlling common oral infections.
Therefore, this study aimed to investigate tomato leaf ethanolic extract’s antioxidant and growth inhibitory capacity against common oral pathogenic microorganisms, namely, Streptococcus mutans, Porphyromonas gingivalis, and Candida albicans.
Materials and methods
Sampling procedure
Leaf samples of ‘Chonto’ tomato (L. esculentum) were collected in a commercial crop in Rionegro, Antioquia, Colombia. The crop had approximately 4,000 plants, was protected with mesh netting, maintained between 18-24°C, and supplied using an irrigation system. Non-chemical methods were used to control pests and diseases.
The foliar tissue was collected in the morning, using gloves, when the flowers began to open and were stored and transported to the CECIF (Center for Pharmaceutical Science and Research, Sabaneta, Antioquia, Colombia) laboratory for further analysis in airtight plastic bags. There, primary asepsis of the leaves was performed with 0.3% sodium hypochlorite to eliminate traces of contaminating substances from their surface. Finally, the physically damaged leaves were discarded.
Preparation of ethanolic extract
The selected and disinfected leaves were dried in a convection oven for 24 h at 45°C and pulverized using a mortar and pestle. Twenty grams of the pulverized material were mixed with 500 mL of absolute ethanol. Then, without artificial light, a mechanical disruption was performed with Ultra-Turrax® (T 25 digital, IKA, Staufen, Germany) (five 1-min cycles at 12,000 rpm with 1-min rest between cycles). The resulting mixture was stored for two weeks in an amber bottle in the dark at room temperature with periodic manual agitation.
Later, the mixture was filtered through a 0.42 μm filter paper to remove particulate matter. An evaporation process was then carried out at 98 mbar, 40°C, and 110 rpm for three h, using a rotary evaporator (Büchi Heating Bath B-490, Buchi Rotavapor R-205).
The extract was transferred to a previously weighed, partially aerated amber glass container with a pipette and stored in the extraction cabinet for two weeks to guarantee complete ethanol evaporation. Finally, 4.27 grams of a viscous, lumpy consistency and dark green color ethanolic extract were obtained. The extract was kept in a closed bottle in the dark at room temperature for later use.
Microorganisms and culture conditions
Bacterial strains S. mutans ATCC® 25175™ and P. gingivalis ATCC® 33277™, and fungal strain C. albicans ATCC® 10231™ were purchased from MDM Científica (Medellín, Antioquia, Colombia).
In a vertical laminar flow cabinet (BIOBASE Meihua Trading Co, model BBS-DDC, Shandong, China), three tubes were served with 10 mL Brain Heart Infusion (BHI) agar (for S. mutans and P. gingivalis) and Yeast Malt (YM) broth (for C. albicans). The tubes were incubated with constant shaking at 150 rpm and 37°C for 24 h. At the end of the incubation, aliquots were taken in sterile 1.5 mL tubes to which 15% glycerol was added, and they were stored at -80°C for later use. Finally, the cultures of S. mutans and C. albicans were maintained in Tryptic soy agar and YM broth at 37°C in aerobic conditions. In contrast, the P. gingivalis culture was maintained in anaerobiosis at 37°C in blood agar. This process and the antimicrobial and antioxidant evaluations were conducted at the Biology Laboratory of CES University.
Antimicrobial assays
We followed the standards on antimicrobial susceptibility testing of bacteria 16 with some modifications. First, the inoculum of each bacterium was prepared by making a direct suspension in the Mueller-Hinton broth of a colony isolated from one of the agar cultures. Next, suspensions were incubated for 24 h at 37°C and 150 rpm, and their absorbance at 600 nm was adjusted to 0.08. Finally, the inoculum was diluted in the plate until it reached an absorbance of 0.04 10.
The antimicrobial activity reading was performed after 24 h of incubation using a spectrofluorometer (Cytation 5, BioTek, Santa Clara, CA, USA) consisting of a 96-well ELISA-type plate reader and a monochromator. The microplates containing the cultures exposed to the tomato extract were placed in the plate reader, and the absorbance at 600 nm was registered.
We followed the standards on antifungal susceptibility testing of yeasts 17 with some modifications. First, the inoculum was prepared by making a suspension in YM broth from one of the tubes stored at -80°C and incubated for 24 h, shaking at 150 rpm and 37°C. Then, the suspension was adjusted to an absorbance of 0.1 at 450 nm. Finally, the inoculum was diluted in the plate until it had an absorbance of 0.05 10.
The antifungal and antimicrobial activity was determined by obtaining the percentage of growth inhibition for each concentration of the extract, using the following equation:
The antifungal fluconazole (MK® 200 mg, Tecnoquímicas, Medellín, Colombia) was used as a positive control against C. albicans, according to the Performance Standards for Antifungal Susceptibility Testing of Yeasts (Clinical & Laboratory Standard Institute). Conversely, the antibiotic vancomycin (Vanbiotic® 500 mg, Vitalis, Bogotá, Colombia) was used as a positive control for S. mutans and P. gingivalis18.
Fluconazole and vancomycin were diluted in dimethyl sulfoxide (DMSO), while the extract was diluted in absolute ethanol. The concentrations used to evaluate the antifungal activity (fluconazole and tomato leaf extract) were 5, 10, 20, 40, 60, 80, 100, and 120 mg/L. On the other hand, the concentrations used to evaluate the antimicrobial activity (vancomycin and tomato leaf extract) were 0.1, 0.25, 0.5, 10, 20, 250, 500, and 1000 mg/L. We evaluated lower, intermediate, and higher values compared to the Clinical and Laboratory Standards Institute (CLSI) guidelines range.
To qualitatively evaluate the growth inhibitory capacity of the extract on S. mutans y P. gingivalis, 20 µL samples of 10, 20, 50, 500, and 1000 ppm were poured in Muller-Hinton agar. Vancomycin (800 ppm) and DMSO were used as controls, and the Petri dishes were incubated for 48 h at 37 °C.
Antioxidant assay
The oxygen radical absorbance capacity (ORAC) assay was utilized to measure the antioxidant capacity of the tomato leaf ethanolic extract using 96-well flat-bottom black microplates. We used 2,2’-azobis(2-amidinopropane) dihydrochloride (AAPH) as an oxidant, Trolox® as a control antioxidant, and fluorescein (FL) as a fluorescent indicator. The protection grade was quantified with a fluorometer (Cytation 5, BioTek). The dissolutions were prepared the same day of the assay and kept in the dark.
Twenty-five μL of the antioxidant solution (Trolox® or tomato leaf ethanolic extract) was added to each microwell. First, to determine the natural oxidation of FL, 25μL of buffer solution was added. Then, 150 μL of the solution, 40 nM, was added in FL. The solution was agitated and incubated at 37ºC for 10 min. Next, 25μL of the solution, 700 mM, was added in AAPH, and the fluorescence reading of the well was registered for 34 min, at 2-min intervals. The fluorescence was measured with 495 nm excitation and 520 nm emission. The microplate was protected from direct exposure to light and the time of adding the oxidant (AAPH).
Statistical analysis
We obtained the dose-response plots and determined inhibitory concentrations and IC50 doses using nonlinear regression. In addition, normality was verified with the Shapiro-Wilk test, the mean difference was analyzed with the Kruskal-Wallis test, and the origin of the differences between concentrations and samples was analyzed with Tukey's multiple comparisons test. A P-value <0.05 was considered significantly different at a 95% confidence level. We used the statistical software GraphPad Prism (San Diego, CA, USA), version 9. Each extract concentration had five biological replicates and three repetitions.
Results
Growth inhibitory activity on S. mutans
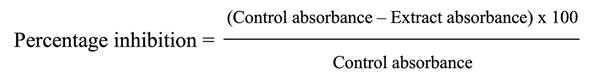
We observed inhibition halos in the tomato leaf extracts, whose area increased according to the concentration. However, vancomycin had the most prominent inhibition halo. As for DMSO, an inhibition halo was observed, but its area was notably smaller than the extracts.
Moreover, the percentage of inhibition was 90% using an extract concentration of 20 mg/L. However, no significant increase was observed when using extract concentrations of 20-500 mg/L, after which a percentage of inhibition of 100% was reached. At lower concentrations (10, 0.5, 0.25, and 0.1 mg/L), no percentage of inhibition was observed (Figure 1).
Growth inhibitory activity on P. gingivalis
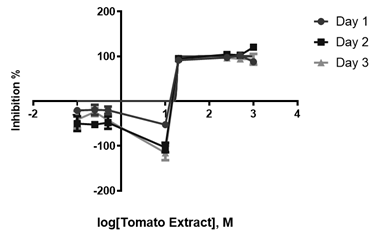
Here, we also observed inhibition halos in the tomato leaf extracts; however, the halos did not increase according to changes in concentration. Vancomycin also had the most significant inhibition halo, and the DMSO halo was imperceptible.
In contrast to S. mutans, we observed high percentages of inhibition (94%) from a lower concentration (10 mg/L). No significant increase was seen at higher concentrations (500 and 1000 mg/L). The highest percentages of inhibition (>100%) were obtained at a concentration over 500 mg/L. At lower concentrations (0.5, 0.25, and 0.1 mg/L), no percentage of inhibition was observed (Figure 2).
Growth inhibitory activity on C. albicans
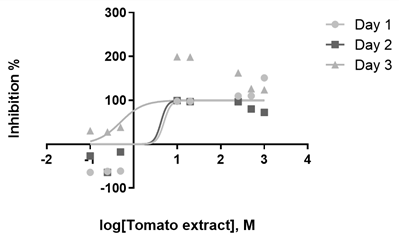
The YM broth pre-inocula obtained an optical density of 0.596 at 450 nm after 24 h at 150 rpm and 26°C. Notably, the highest percentage of inhibition on C. albicans was 37% for the extract and 83% for fluconazole (Figure 3). The EC50 values of the evaluated compounds are described in Table 1.
Antioxidant capacity
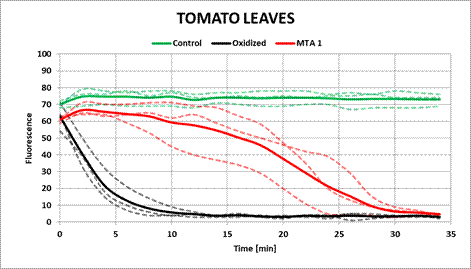
The tomato leaf ethanolic extract obtained an antioxidant capacity of 126%. As is shown in Figure 4, the FL indicator preserves its absorbance over time, indicating that the extract protects the FL from oxidation for 30 min, compared to 5 min, after which FL oxidates in the absence of an antioxidant.
Discussion
To our knowledge, this is the first study to investigate the antimicrobial potential of tomato leaf extracts against P. gingivalis and S. mutans. We found a high percentage of growth inhibition (≥100%) against these bacteria at a concentration of 500 mg/L. Only one study, conducted in Nigeria, has previously evaluated the antimicrobial effect of tomatoes against S. mutans19. The authors reported that aqueous and methanolic extracts had no inhibitory effect on this microorganism at various concentrations (12.5, 25, 50, and 100 mg/mL). However, the extracts were made from the fruit, which could explain the divergence from our findings11. Another study analyzing the antimicrobial capacity of leaves, stems, roots, and whole-plant extracts of two tomato varieties, i.e., Pitenza and Floradade, found a significant activity against Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, and Listeria ivanovii. Interestingly, the leaf extracts showed the highest antimicrobial activity in both varieties, followed by the stem and whole-plant extracts4. Similarly, a recent study found that leaf extracts exhibited the highest growth inhibition against Bacillus cereus and Salmonella enterica, as well as Trichomonas vaginalis, compared to stem, peel, and fruit extracts11. Finally, based on the existing reports, it is evident that Gram-positive bacteria (e.g., S. aureus) are more sensitive to tomato extracts than Gram-negative bacteria10,20,21, which is in line with our results. It has been hypothesized that the lipopolysaccharides content in the external cell membrane of Gram-negative bacteria could provide higher resistance to the antimicrobial compounds of the tomato plant10,21.
In this study, we also investigated the antifungal activity of tomato leaf extract against C. albicans -an opportunistic pathogenic yeast that causes oral and vaginal candidiasis- and found that it was ineffective in inhibiting its growth. However, the strain used in our study likely presented with resistance genes, as the percentage of inhibition was 37% for the extract and only 83% for fluconazole. The few studies that have been published on the antifungal activity of tomato extracts show conflicting results. Tomato leaves, stems, and green tomato fruit inhibited the growth of C. albicans to a significant extent (63-74%) relative to the positive control octyl gallate11. Furthermore, another study showed that tomato seed extracts displayed antifungal properties, although they seemed solvent-dependent and less effective than against Gram-positive bacteria21. Of note, all the five different extracts tested (i.e., chloroform, methanol, ethyl acetate, hexane, and sulfuric acid) were active against C. albicans, except for the ethyl acetate extract. Other studies have found no growth-inhibiting activity of tomato fruit aqueous and methanolic extracts against C. albicans19,22. Hence, the contrasting results might be attributed to the solvents used in the extraction protocols, as it has been shown that the solubility of antimicrobial and antioxidant compounds in tomato extracts largely depends on the solvent polarity23. In general, organic extracts yield a more potent antimicrobial activity due to the presence of non-polar residues in the solvent compared to aqueous extracts, which tend to have lower bactericidal and bacteriostatic properties19,24.
Finally, we observed that the tomato leaf extract exerted high antioxidant activity compared to the positive control (Trolox®), which is in line with previous results4,10,20. The primary antioxidants that have been identified in tomato fruit, leaf, and waste (peel and seed) extracts include phenolic compounds, flavonoids, carotenoids (e.g., β-carotene, α-carotene, β-cryptoxanthin, lycopene, and lutein), and chlorophyll4,10,20,25. For instance, Silva-Beltrán et al. measured the antioxidant capacity of stem, root, leaf, and whole-plant extracts of two tomato varieties using the ORAC assay. The leaf extracts exhibited the highest antioxidant activity in both varieties, which correlates with the high content of phenolic compounds (especially gallic acid, chlorogenic acid, and rutin), flavonoids, and chlorophyll in the leaves compared to other parts of the plant4. However, the total antioxidant activity of an extract results from the interaction between several constituents, which may include synergistic or additive effects26.
Identifying and quantifying bioactive compounds of tomato leaf extracts were beyond the scope of this study, which might be considered a limitation. However, antimicrobial and antioxidant metabolites in different parts of the tomato plant have been widely investigated. As the results of potent antioxidants, some studies have shown that the leaves are the primary source of bioactive compounds with antimicrobial properties, such as glycoalkaloids (i.e., tomatine and tomatidine), phenolic compounds, terpenoids, and flavonoids4,11,27. The primary antimicrobial action of some of these compounds, such as phenols, flavonoids, and terpenoids, is conferred by the presence of hydroxyl groups, which destroy the cell membrane of bacteria, causing the leakage of cellular components. Moreover, these hydroxyl groups can act in the active site of enzymes and damage the metabolic processes of microorganisms28. The free hydroxyl groups in phenolic compounds and flavonoids also act as antioxidants, as they inhibit the generation of reactive oxygen species and scavenge free radicals, thereby reducing the redox potential of the growth medium29.
Conclusions
This study provides new insights into the potential antimicrobial effect of tomato leaf extracts on common oral pathogenic bacteria, which may ultimately result in the development of new herbal products that might help prevent and treat oral infections, such as dental caries and periodontal disease. Our findings also support previous studies on the high antioxidant capacity of tomato leaf extracts.