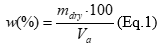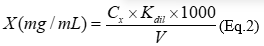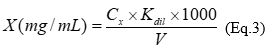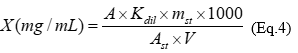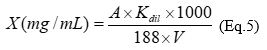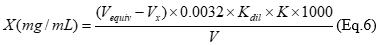Introduction
Nowadays we observe a great interest in antioxidants, especially nature origin, due to the increasing level of cancer, cardiovascular and metabolic diseases 1. Those diseases are associated with the growing impact on humans of adverse environmental factors, such as ultraviolet and electromagnetic radiation, man-made pollution of the atmosphere with radioactive and toxic compounds, the use of food products with a high content of artificial chemical agents (preservatives, thickeners, dyes, flavors, etc.), leading to the formation of an excess amount of free radicals in the body and oxidative damage to organic macromolecules 2.
Oxidative stress causes an imbalance of the natural antioxidant system, significantly increases the risk of developing diabetes mellitus, atherosclerosis, cancer, heart ischemia, and Alzheimer's disease. Therefore, it is recommended to regularly consume certain foods and drinks, drugs, dietary supplements with antioxidant activity to neutralize the harmful effects of free radicals 3.
Raspberry (Rubus idaeus L.) is a shrub that belongs to the Rosaceae family and widely grows in Ukraine, Europe, Asian and America 4. Raspberry fruit, leaves, and shoots have been used for medicinal purposes for centuries 5,6. The leaves and fruits were traditionally used to treat gastrointestinal, respiratory disorder and heart problems. Raspberry shoots were used directly to treat sore throat, flu, fever, and diabetes 7.
Red raspberry shoots contain catechins, ellagitannins, flavanol derivatives, ellagic acid, phenolic, and organic acids 8. Ellagotannins are a group of hydrolyzable tannins characteristic of the Rosaceae family. Sanguine H-6 is the main ellagitannin found in raspberry shoots 9. Raspberry leaves have also been found to contain ellagitannins, some catechins and flavanols, and organic acids 10,11. In contrast to the shoots and leaves of red raspberries, eleven anthocyanins were found in the fruits 12,13.
The antioxidant activity of raspberry fruit and leaves have been established 5,14. However, much less information is available on the antioxidant activity and chemical composition of raspberry shoots extracts. There is no reviews about the determination total antioxidant capacity of raspberry shoots. The total antioxidant capacity of raw materials needs to be studied and further apply total antioxidant in developing drugs, dietary supplements and cosmetologically products.
This study aimed to determine the total antioxidant capacity and biologically active substances content of red raspberry shoots extracts obtained by subsequent exhaustive extraction.
Materials and methods
Plant material
The leafless second-year red raspberry shoots were collected after the fruiting period near village of Ternova, Kharkiv region, Ukraine in 2021.
Equipment and reagents
The pH meter HANNA 2550 (Germany) with a combined platinum electrode EZDO 50 PO (Taiwan) was used for potentiometric measurements. Quantitative analysis of biologically active compounds was carried out on UV-spectrophotometer UV - 1000 (China) with matched 1 cm quartz cells. The weighing was performed using digital analytical balance АN100 (AXIS, Poland) with d = 0.0001 g. All solvents and other chemicals used in the study were of analytical grade.
Extraction procedure
A 10.0 g of red raspberry shoots were ground to 1-2 mm in size. The extraction was carried out one by one using distilled water, 20% ethanol, 40% ethanol, 60% ethanol, and 96% ethanol at the ratio raw material/solvent 1/20 (m/v) in a water bath at 80º C with reflux for 1 hour. After cooling, the solutions were filtrated and concentrated to 20 mL by a rotary evaporator at 40 º C under a vacuum.
Quantitative analysis
2.0 mL of the extract was placed in a weighing bottle, brought to a constant mass, evaporated in a water bath, and dried from 100 to 105 ° C for 3 hours. The weighing bottle was cooled in a desiccator at room temperature for 30 min and weighed 16. The dry residue w, (%) in extract samples was calculated according to equation 1:
where, mdry - mass of the dry residue after drying an aliquot of the extract sample, g; Va - volume of extract sample aliquot, mL.
The total content of phenolic compounds was measured by the Folin-Ciocaltau assay, the absorbance was measured at 760 nm 15. The phosphomolybdotungstic reagent was used as standard. The calibration curve (Y = 0.1055X + 0.1745 (R2=0.9951)) was plotted with interval concentrations 1.0 - 5.0 μg/mL, the calibration equation. The total phenolic compounds content in extracts (X), expressed as GA was calculated according to equation 2:
where, С x - concentration of gallic acid according to the calibration curve, С×10-6, g/mL; V - extract volume, mL; K dil - coefficient of dilution, mL.
The vanillin reagent assay was applied to determine the total catechins 18; the absorbance was measured at 505 nm. The calibration curve (Y = 0.0025X - 0.0851 (R2 = 0.9951)) was plotted with 100 - 400 μg/mL interval concentrations of epigallocatechin-3-O-gallate (EGCG). The total catechins content in extracts (X), expressed as EGCG was calculated according to equation 3:
where, С x - concentration of epigallocatechin-3-O-gallate according to calibration curve, С×10-6 g/mL; V - volume of extract, mL; K dil - coefficient of dilution, mL.
The total flavonoids were determined using the complex formation assay with AlCl3; the absorbance was measured at 417 nm 19. The concentration of standard solution of rutin was 0.02 mg/mL. The total flavonoids content in extracts (X), expressed as R, was calculated according to equation 4:
where, A - absorbance of analyzed solution; A st - absorbance of standard solution of rutin; V - volume of extract, mL; K dil - coefficient of dilution, mL, mst - mass of rutin, g.
The total hydroxycinnamic acids derivatives content was measured by assay of complex formation with NaNO2-Na2MoO4, the absorbance was measured at 525 nm 20. The total content of hydroxycinnamic acids derivatives in extracts (X), expressed as chlorogenic acid (ChA) was calculated according to equation 5:
where, A - absorbance of analyzed solution; 188 - specific adsorption coefficient of chlorogenic acid; V - volume of extract, mL; Kdil - coefficient of dilution, mL.
The total organic acids content was determined by acid-base titration with the fixation endpoint by potentiometric method 21. The total content of organic acids in extracts, expressed as citric acid (CA) was calculated according to equation 6:
where, 0.0032 - the amount of citric acid, equivalent to 1 mL of sodium hydroxide solution (0.05 mol/L), g; Vequv. is the volume (mL) of sodium hydroxide solution (0.05 mol/L), which was used for titration; Vx - the volume (mL) of sodium hydroxide solution (0.05 mol/L), which was spent for titration in a blank experiment; V - volume of extract, mL; Kdil - coefficient of dilution, mL.; K is the correction coefficient for 0.05 mol/L sodium hydroxide solution.
Antioxidant activity assay
Antioxidant activity (AOA) of extracts was evaluated by potentiometric method 22. Antioxidant activity was calculated according to equation 7 and expressed as mmol-equiv./mdry res.:
where, α = C ox/C red × 10 (∆E - Eethanol)nF/2.3RT ; С ox - concentration of K3[Fe(CN)6], mol/L; C red - concentration of K4[Fe(CN)6], mol/L ; Е ethanol - 0.0546·С% - 0.0091; С % - concentration of ethanol; ∆E - change of potential; F = 96485.33 C/mol - Faraday constant; n = 1 - number of electrons in electrode reaction; R = 8.314 J/molК - universal gas constant; T - 298 K; K dil- coefficient of dilution, mL.; m 1 - mass of dry residue; m 2 - mass of dry residue in 1.0 mL of extract.
Correlation analysis
Pearson’s (r) correlation coefficient was used to analyze the correlation between antioxidant activity (AOA) and the amount of phenolic, catechin, flavonoid, hydroxycinnamic acids derivatives and organic acids. The correlation coefficient to takes a value in the range of -1 to +1. Correlation is very high if it is within the range from 0.90 to 1.00; from 0.70 to 0.90 is a high correlation; from 0.50 to 0.70 is a moderate correlation; from 0.30 to 0.50 is a low correlation; from 0.00 to 0.30 negligible correlation 23.
Results
Determination the total content of phenolic compounds
The aqueous extract had the most significant content of phenolic compounds (13.90±0.29 mg/mL), while other raspberry shoots extracts demonstrated a much lower content of phenolic constituent (Table 1). The sum of the total phenolic compound content of red raspberry shoots extracts was 24.40 mg/mL.
Determination the total content of catechins
The sum of total catechins content was 21.36 mg/mL. The highest amount of catechins was observed in the aqueous extract (12.50±0.25 mg/mL), followed by the other ethanol extracts. According to the results, the amount of catechins (87.54 %) was higher among all phenolic compounds (3.16 % flavonoids, and 1.49 % hydroxycinnamic acids derivatives) (Table 1).
Determination the total content of flavonoids
The sum of total flavonoid content was 0.77 mg/mL. The highest amount of flavonoids was observed in the aqueous extract (0.40±0.01 mg/mL), followed by the other ethanol extracts (Table 1).
Determination the total content of hydroxycinnamic acids derivatives
The sum of total hydroxycinnamic acids derivatives content was 2.56 mg/mL. The highest amount of hydroxycinnamic acids derivatives was observed in the aqueous extract (1.50±0.03 mg/mL), followed by the other ethanol extracts (Table 1).
Determination the total content of organic acids
The aqueous extract had the greatest content of organic acids (1.10±0.03 mg/mL), while other raspberry shoot extracts demonstrated a much lower content of organic acids. The sum of total organic acids content was 1.88 mg/mL (Table 1).
3.6. Determination the total antioxidant activity
The total antioxidant capacity of raspberry shoots was 164.12 mmol-equiv./mdry weight. The antioxidant activity increases in the following extracts order: 96 and 80 % < 60 % extract < 40 % extract < 20 % extract < aqueous (Table 1).
Table 1 The quantitative content of catechins, flavonoids, hydroxycinnamic acids derivatives, organic acids, and antioxidant activity, calculated from the extraction of red raspberry shoots (1:20 ratio raw material/solvent)
| Extractant | Total phenolic content, mg/mL | Total catechin content, mg/mL | Total flavonoid content, mg/mL | Total hydroxycinnamic acids derivatives content, mg/mL | Total organic acids, mg/mL | Antioxidant activity, mmol-equiv./mdry weight |
| distilled water | 13.9±0.29 | 12.5±0.25 | 0.40±0.01 | 1.50±0.03 | 1.10±0.03 | 85.00±1.70 |
| 20% ethanol | 5.8±0.12 | 5.0±0.10 | 0.20±0.01 | 0.60±0.01 | 0.40±0.01 | 37.60±0.75 |
| 40% ethanol | 4.1±0.10 | 3.4±0.07 | 0.14±0.01 | 0.40±0.01 | 0.30±0.01 | 37.26±0.75 |
| 60% ethanol | 0.4±0.01 | 0.3±0.01 | 0.02±0.003 | 0.04±0.003 | 0.03±0.003 | 3.11±0.06 |
| 80% ethanol | 0.1±0.001 | 0.08±0.003 | 0.005±0.001 | 0.01±0.001 | 0.01±0.001 | 0.62±0.01 |
| 96% ethanol | 0.1±0.001 | 0.08±0.001 | 0.005±0.001 | 0.01±0.001 | 0.01±0.001 | 0.62±0.01 |
| The total content | 24.40 | 21.36 | 0.77 | 2.56 | 1.88 | 164.12 |
Correlation analysis
Pearson’s (r) coefficients between antioxidant activity and phenolic compounds, catechins, flavonoids, hydroxycinnamic acids derivatives, and organic acids were 0.9898, 0.9860, 0.9723, 0.9851 and 0.9697, respectively (Table 2).
Table 2 Pearson`s (r) correlation coefficient between antioxidant activity and biologically active compounds content in extracts of red raspberry shoots
| Total phenolic content | Total catechin content | Total flavonoid content | Total hydroxycinnamic acids derivatives content | Total organic acids content | |
| Antioxidant activity | 0.9898 | 0.9860 | 0.9723 | 0.9851 | 0.9697 |
Discussions
Description of sequential exhaustive extraction
In our previous research 23, we created and described the approach of exhaustive sequential extraction. This type of extraction is based on the extraction of the same raw material with extractants of different polarity for example, distilled water as a more polar extractant and ethanol solutions of different concentrations as a less polar extractant. Consistent, exhaustive extraction allows you to extract hydrophilic and lipophilic BAS completely. In this case, the raw material was not dried after extraction; therefore, the volume of the extractant absorbed by the raw material was considered.
Determination the total content of phenolic compounds
Raspberry shoots are a rich source of phenolic compounds such as ellagitannins, catechins, flavonols and phenolic acids derivatives. Ellagitannins are a group of complex phenolic compounds found in high concentrations in raspberry shoots. They are represented by the derivatives of sanguiin and lambertianin. Ellafitannins are metabolized in the gut to form ellagic acid, which has been shown to have anti-inflammatory and anticancer properties 8. The Folin-Ciocalteu method was applied to evaluate the total amount of phenolic compounds. The total phenolic compounds were expressed as gallic acid. A recent study of Bobinatie R. et al.25, they have investigated 41 red raspberry leaves cultivars. They found that the phenolic compounds content varied in different samples from 1.0 to 6.0 mg/mL in ethanolic extract. Compared to our results, in our study, the total amount of phenolic compounds is higher from 4 to 24 times. In another study, Pavlovic et al. reported 144.20 mg/mL in the 60% methanolic extract of red raspberry leaves 26. Buricova et al. evaluated a total content of phenolic compounds of 2.76 mg/mL aqueous extract of red raspberry leaves 27. In our opinion, the difference in the content of phenolic compounds, is associated with different brewing times, leaves/extractant ratios used, species, climate, and geographical position.
Determination the total content of catechins
The raspberry shoots presented a variety of catechins, such as epigallocatechin-3-O-gallate, epicatechin, catechin, and epigallocatechin. The catechin is the main compound among catechins in the raspberry shoots 28. The vanillin reagent assay determined the total catechins expressed in epigallocatechin-3-O-gallate equivalent. In recent research by Durgo K. et al.9, the total catechins found were 0.17 mg/mL in the aqueous extract of red raspberry leaves in our research, we are reporting 21.36 mg/mL, 120 times higher than obtained by the authors. Luo et al. reported a total catechin content of 0.10 mg/mL in 60% methanolic extract red raspberry leaves 29, and we obtained 0.3 mg/mL in ethanol extract. The large values difference may be because that raspberry shoots contain more catechins than flavonol derivatives. Catechins in the biometabolism of plant shoots are responsible for the intensity of cell division and protection. In contrast, flavonol derivatives have a signal function and remarkable antioxidant effect against reactive oxygen species.
Determination the total content of flavonoids
Flavonoids are a phytochemical widely distributed in raspberries with various health benefits. Several flavonols have been detected in the raspberry shoot: rutin, quercetin-3-O-glucuronide, hyperoside, myricetin and kaempferol 8. The total flavonoids were determined using complex formation with AlCl3 assay. The total flavonoid content was expressed in rutin equivalent. Costa T. et al.30, reported 12.92 mg/mL of flavonoids in 20% ethanolic extract of red raspberry leaves. In contrast, we obtained a value of 94 % lower (0.20 mg/mL). Pavlovic et al. reported a total flavonoids content of 10.64 mg/mL in 60% methanolic extract red raspberry leaves extract 26. Comparing the content of flavonoids in extracts obtained from the leaves and shoots of red raspberry, we can conclude that flavonol derivatives dominate in the leaves, then in the shoots. Therefore, our theory mentioned above is approved.
Determination the total content of hydroxycinnamic acids derivatives
Raspberries are a rich source of hydroxycinnamic acids. The most abundant hydroxycinnamic acid in raspberries is chlorogenica acid, a powerful antioxidant associated with various health benefits. Other hydroxycinnamic acids in raspberries include: caffeic acid, p-coumaric acid, ferulic acid and sinapic acid 9. The total hydroxycinnamic acids derivatives content was measured by assay of complex formation with NaNO2-Na2MoO4 and expressed in chlorogenic acid equivalent. Costa T. et al.30 reported 9.28 mg/mL hydroxycinnamic acids derivatives in 20% ethanolic extract of red raspberry leaves. in our case, the total amount of hydroxycinnamic acids derivatives was 72.11% lower. Yang et al. reported a total hydroxycinnamic acids content of 8.24 mg/mL in distilled water red raspberry leaves extract 31, meanwhile, we obtained the total hydroxycinnamic acids - 2.56 mg/mL. We have observed that the concentration of hydroxycinnamic acids in raspberry leaf extract is higher than in the shoot extract. We believe that this is related to the biometabolism of flavonoids. According to the shikimate pathway, derivatives of hydroxycinnamic acids serve as precursors to flavonoids. As we have also demonstrated that the concentration of flavonoids is higher in the leaf extract than the shoot extract, it follows that the content of hydroxycinnamic acids in the leaf extract should also be higher.
Determination the total content of organic acids
Some of the main organic acids in raspberry shoots include malic acid, citric acid, quinic acid, and oxalic acid and citric acid is considerably abundant in raspberry shoots 32. The total organic acids content was determined by acid-base titration with the fixation endpoint by potentiometric method. The total organic acids were expressed in citric acid. Mikulic-Petkovsek M. et al.33 investigated amount of organic acids in the aqueous extract of red raspberry fruits by high-performance liquid chromatography. This study revealed that the total organic acids were 2.2 mg/ml In our research, the sum of total organic acids was 14.54 % lower than in the reported study.
Determination the antioxidant activity
The antioxidant activity values of investigated extracts were estimated with the potentiometric method. This method was chosen due to its high sensitivity, rapid analysis procedure, and relatively low cost of equipment and reagents 34. However, certain acceptance criteria are required to justify the choice of extraction conditions,. According to the available literature, the main acceptance criterion is obtaining the maximum content of phenolic and extractive compounds. We propose to use the total antioxidant capacity of raw materials as an acceptance criterion for choosing the optimal extraction conditions, for several of reasons, firstly, the antioxidant activity and the content of phenolic compounds have a high positive correlation, secondly, the determination of antioxidant activity requires less time than the determination of the content BAS and extractive compounds. The term "total antioxidant capacity of raw materials" means the sum of the antioxidant activity of all hydrophilic and lipophilic BAS contained in the studied raw materials.
Correlation analysis
Luo et al. 29 reported a good correlation between the antioxidant activity of red raspberry extract and the amount of phenolic compounds. They found correlation coefficients of 0.915 and 0.830 when evaluating antioxidant activity by ABTS and DPPH assays, respectively. In our study, the highest correlation coefficient value was between antioxidant activity and the total content of phenolic compounds (r = 0.9898). Therefore, phenolic compounds influenced the antioxidant activity of red raspberry leaf extract.
Conclusions
The total red raspberry shoots antioxidant activity was determined. The BAS and antioxidant activity analysis of red raspberry shoot extracts revealed that the aqueous extract had a remarkable content of phenolic compounds, catechins, flavonoids, hydroxycinnamic acids, organic acids and antioxidant activity. Moreover, the quantitative analysis showed that catechins were the main compounds among phenolic ones. Furthermore, correlation analysis revealed a strong positive linear relationship between antioxidant activity and phenolic compounds. The study results can be used to develop optimal technologies for obtaining drugs based on the extract of red raspberry shoots, which has an antioxidant effect.