Introduction
In metabolism, various enzymes act as biocatalysts that interact with foreign substances. In some cases, enzymes are known to act as drug targets 1 for human diseases. Treating these diseases with synthetic enzyme inhibitors often fails due to toxicity and side effects. Therefore, it is necessary to search for non-cytotoxic and effective enzyme inhibitors agents from natural sources 2.
Traditional herbal medicine has extensively used different plant parts to treat various disorders such as cardiovascular, gastrointestinal, diabetes, wound healing, and microbial infections. 3-8. Moringa peregrina (Forssk) Fiori is a well-known herbal plant, and the wide range of pharmacological properties of its seeds have been reported, including immunomodulatory effect, antioxidant, antimicrobial activity, analgesic, and cytotoxic effects against several cancer cell lines 9,10, which can be exploited in the development of drug candidates. The seeds of M. peregrina are traditionally used to control diabetes, wounds, gastrointestinal diseases, anti-inflammatory, and anticancer 11,12. Some active phytochemicals in M. peregrina (triterpenoids, tocopherols, flavonoids, isothiocyanate, quercetin, and phenolics) have been well-reported to enhance the immune system to fight against cancer cells 10,13-15.
In type II diabetes mellitus, cells resist insulin, and glucose is not properly utilized, leading to hyperglycemia. The α-glucosidase inhibitors have been proposed as a promising approach to treating diabetes. A drug that inhibits α-glucosidase activity prevents disaccharides’ breakdown into monosaccharides, lowering blood glucose levels. This approach causes a decrease in glucose absorption from the gastrointestinal tract, preventing the rise in glucose levels after food consumption. Therefore, researchers are looking for plant-based products with anti-α-glucosidase properties 16.
Thymidine phosphorylase (TP) is an enzyme responsible for the initiation of angiogenesis. Hyper activation of thymidine phosphorylase is involved in tumor development by promoting endothelial cell migration and the release of various angiogenic factors from malignant and stromal cells in the tumor microenvironment site and helps in the evasion of apoptosis and immune cells. Therefore, it is necessary to discover therapeutic agents that can stop angiogenesis 17.
Carbonic anhydrase is a well-known drug-target enzyme responsible for physiological processes related to respiration and the transport of CO2/bicarbonate between metabolizing tissues and the lungs. Carbonic anhydrase is involved in biosynthetic reactions such as bone resorption, calcification, gluconeogenesis, lipogenesis, and ureagenesis. Carbonic anhydrase enables tumor growth compared to normal tissues 18.
The rapid development of resistance in cancer cells to prevailing anticancer drugs and ineffective surgical or radiotherapy had often failed to reduce the chances of recurrence and ultimately led to the progression of cancer to the metastatic stage 19,20. In the recent decades, researchers have paid attention to natural products as active compounds isolated from plants that are considered safe and have pharmacological anticancer effects 21,22.
The current research was the first-time report on the effect ofM. peregrina seed extract on the enzyme inhibition potential for α-glucosidase, thymidine phosphorylase, and carbonic anhydrase. Furthermore, the anticancer potential of M. peregrina seed extract was investigated in the human neuroblastoma cell line SH-SY5Y.
Materials and methods
Plant Preparation
M. peregrine seeds were submitted and authenticated by Dr. Maha Kordofani (Resident Botanist) at the Department of Botany, University of Khartoum. M. peregrine seeds were kept at room temperature until they were dried and ground to powder. 100 g of powder was mixed s with 500 mL of ethanol and left for 5 days for proper mixing. The macerates were filtered through a muslin cloth and collected; then, rotary evaporated to obtain the crude M. peregrine seed extract (MPSE) 23.
Alpha-Glucosidase inhibition assay
20 µL of MPSE prepared in different concentrations (0.125-1 mg/mL) were added to a 96-well plate. Along with the test extract, 20 µL of enzyme solution (0.2 U/mL) and 135 µL of 0.1 M phosphate buffered saline (PBS) buffer (pH 6.8) were added to each well. The plate was then incubated at 37 °C for 15 min. After incubation, the absorbance was measured before the substrate addition. The substrate (25 µL) 4-nitrophenyl-α-D-glucopyranoside (PNP-G) (0.7 mM) was then added, and the change in absorbance was measured for 30 min at 400 nm in a spectrophotometer (Spectra Max, MD, USA) 24. Acarbose served as the positive (drug) control.
Thymidine phosphorylase (TP) inhibition assay
We followed the protocol of Javaid et al. 25. Briefly, 10 µL of MPSE at different concentrations (0.125-1 mg/mL) were incubated with 20 μL of the enzyme (0.058 unit/well) along with 150 μL of potassium phosphate buffer (pH 7.0, 50 mM) for 10 min at 30°C. After 10 min, 20 μL (1.5 mM) substrate (thymidine, kmax; 265 nm) was added. Soon after, absorbance was recorded at 290 nm using an ELISA plate reader and continued for 10 min to monitor the change in absorbance. 7-Deazaxanthine served as the positive (drug) control.
Carbonic anhydrase inhibition assay
In this assay, 20 µL of different concentrations of MPSE (1-12.5 mg/mL) were added to a well. Along with the test extract, 20 µL enzyme carbonic anhydrase solution (0.1 mg/mL) and 140 µL of 20 mM HEPES-tris buffer (pH 7.4) were added to each well. The 96-well plate was then incubated at 37 °C for 15 min. After incubation, the absorbance was measured, and then 20 µL of the substrate solution 4-nitrophenyl acetate (0.7 mM) prepared in methanol was added to all wells. The amount of product formed was monitored by the change in absorbance for 30 min with intervals of 1 min at 400 nm in a spectrophotometer (Spectra Max, MD, USA) 26. Acetazolamide served as the positive (drug) control.
Cytotoxicity Analysis Using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) colorimetric assay
The anticancer activity of the MPSE at different concentration ranges (1000-10 µg/mL) was measured in the human neuroblastoma cell line SH-SY5Y. These cells were grown in Dulbecco's Modified Eagle Medium (DMEM) media supplemented with 10% fetal bovine serum and incubated in a 5% CO2 incubator at 37°C. Upon 75-85% confluency, cells were detached from the flask and seeded at 5,000 cells/well into 96-well plates. The next day, after adherence of cells on the plate, these cells were treated with MPSE at double-fold diluted concentrations ranging from 100-1 μg/mL. After 24 h, cell viability was checked by adding 20 µL of MTT (5 mg/mL) dye to each well and the plate was incubated for 3-4 h. Soon after, formazan crystal was dissolved by adding 100 µL of 0.1% dimethyl sulfoxide (DMSO). The plate was shaken for 1 min, and absorbance was measured at 540 nm using a spectrophotometer (Tecan, California, USA) 27.
Result
Enzyme inhibitory activity
In the present study, MPSE was tested on different enzyme systems to evaluate its potential to inhibit enzymes that cause chronic diseases. At 0.5 mg/mL, the MPSE revealed anti-enzyme activities against α-glucosidase, thymidine phosphorylase, and carbonic anhydrase with inhibitory percentage values of 89 ± 3, 51.3 ± 0.3, and 80 ± 5 % respectively. The IC50 of inhibitory activity of MPSE is shown in Table 1.
Table 1: Enzyme inhibitory activity of the M. peregrina seed ethanol extract
| Enzyme Activity | IC50 values | ||
| MPSE | a standard | ||
| α-glucosidase | 160.1 ± 5 µg/mL | 875.75 ± 2.08 µM | |
| Thymidine phosphorylase | 500 ± 6 µg/mL | 41.0 ± 1.63 μM | |
| Carbonic anhydrase | 180 ± 5 µg/mL | 18.2 ± 1.23 μM | |
a The standard drug Acarbose was used in α-glucosidase assays, 7-Deazaxanthine in Thymidine phosphorylase, Acetazolamide in Carbonic anhydrase
Anticancer activities
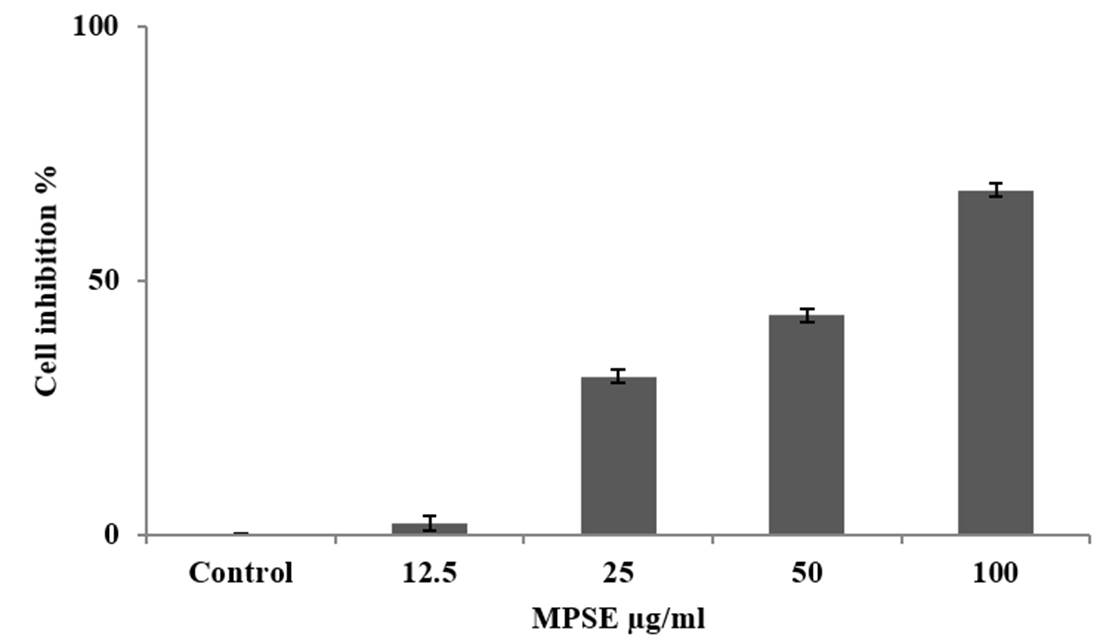
Cytotoxicity was measured by MTT assay. The result showed that MPSE inhibited the growth of cancer cells SH-SY5Y, with IC50values of 55.1 μg/mL (Figure 1).
Discussion
According to the World Health Organization (WHO) report, chronic diseases are a silent global epidemic that accounts for 71% of deaths worldwide 28. Among these chronic diseases, diabetes is one of the major risk factors for causing other diseases related to cardiovascular diseases, respiratory diseases, cancer, neurodegenerative disorders, nephropathy, and neuropathy diseases 29. For the progression of these chronic diseases, enzymes like carbonic anhydrase, thymidine phosphorylase, and α-glucosidase play a vital role in the slow progression of the disease. Therefore, it would be beneficial to inhibit these enzymes with various active compounds. In response to these, researchers have been screening plant extracts against these enzymes to decrease the disease progression with fewer side effects.
This present study concluded that MPSE at 0.5 mg/mL showed enzyme inhibitory activity of 89 ± 3, 51.3 ± 0.3, and 80 ± 5 % against α-glucosidase, thymidine phosphorylase, and carbonic anhydrase, respectively. Sardabi et al. 30 reported a 50% inhibition of α-glucosidase by the defatted M. peregrina seed pressed cake at 30 mg/mL. Ullah et al. 31) reported the potential of the hydro-alcoholic extract of M. peregrina to inhibit α-amylase and α-glucosidase. They attributed these activities to higher concentrations of total phenols, saponins, and tannins. Many active compounds have been reported from M. peregrina, such as polyphenolic compounds, flavonoids, tocopherols, linoleic acid, and oleic acid, which may possess enzyme inhibition activity 32,33. Koheil et al. 34) reported the antidiabetic activity of M. peregrina seeds hydro-alcoholic extract fraction in streptozotocin-induced diabetic rats. This extract significantly decreased the blood glucose level at 200 mg/kg of body weight. The other two fractions (petroleum ether and chloroform) also decrease the blood glucose level.
In many solid tumors, overexpression of thymidine phosphorylase induces angiogenesis and prevents apoptosis of cells leading to the metastatic stage. Due to its critical function, thymidine phosphorylase is an ideal target for discovering anti-angiogenic compounds. In the present study, MPSE showed inhibition of thymidine phosphorylase enzyme with an IC50 of 471 µg/mL.
In solid tumors like neuroblastoma, a hypoxic condition leads to an acidic microenvironment; therefore, to survive in this acidic environment, these continuously dividing cells overexpress carbonic anhydrase IX and XII isoforms enzymes 35. Over expressions of these two isoforms maintains a physiological intracellular pH due to which cells could survive in acidic extracellular pH. Thus, tumors progress to the metastatic stage. Due to the critical role of carbonic anhydrase in the neuroblastoma progression, it is considered a potential biomarker to predict the survivability of neuroblastoma patients 36. Therefore, many researchers have considered carbonic anhydrase inhibition as the targeted therapy. In the present study, M. peregrina seed extract showed promising anti-carbonic anhydrase inhibition with IC50 of 271 µg/mL. Besides, MPSE showed anti-neuroblastoma activity with IC50 of 55 µg/mL. It was reported that carbonic anhydrase inhibitor increases the anti-neuroblastoma effect of cisplatin through a synergistic effect 37; therefore, MPSE combined with cisplatin may show a synergistic effect. Thus, this study paved the way to study the potential of MPSE and its synergistic effect with cisplatin in treating neuroblastoma.
M. peregrina seed extract inhibited three different enzymes: α-glucosidase, carbonic anhydrase, and thymidine phosphorylase. M. peregrina was also reported to possess enzyme inhibition activity against urease 23, dipeptidyl peptidase IV 31, and angiotensin-converting enzyme 38. Thus, this plant could be used in treating different chronic diseases caused due to the overexpression of these enzymes 39.
M. peregrina seeds have been reported for their cytotoxic effect on cell lines HeLa, MCF-7, CaCO2, and HepG2 40. The anti-cancer activity showed by MPSE against these cancer cell lines, and SH-SY5Y might be due to the presence of phenolic/flavonoid compounds 41-44 like catechin, catechol, resveratrol, coumarin, naringin, rutin, quercetin, kaempferol, hispertin, and apigenin, which induce apoptosis by different pathways like activation of the mitochondrial pathway, and caspase-3 dependent apoptotic pathway, downregulation of anti-apoptotic genes (Bcl-2, Bcl-xL), and upregulation of caspase3 with the release of cytochrome c., premature aging, cell cycle arrest, and enhance the immune system to engulf cancer cells 45,46.
Conclusion
M. peregrina seed extract showed good enzyme inhibition against α-glucosidase and carbonic anhydrase with IC50 value below 200 µg/mL. M. peregrina also revealed anti-neuroblastoma activity, which supported its anticancer property against other cancer cell lines. The results showed carbonic anhydrase inhibitor along with anti-neuroblastoma effect of the extract. Therefore, M. peregrina could be combined with available anticancer drugs to enhance their anticancer effects.