INTRODUCTION
The giant African snail (Achatina fulica) is considered one of the 100 most dangerous invasive exotic species worldwide, according to the International Union for the Conservation of Nature. This is due to the intrinsic characteristics of its natural history, such as high reproductive capacity, polyphagia, and the sympathy that it produces in humans, who have used it for several economic purposes 1. When the species began to disperse from its natural distribution range in the African continent towards other continents such as Asia, Europe, and America, it caused numerous economic losses for agriculture and commerce. It also generated great worry because of its impact on public and veterinary health in the places it colonized, because this gastropod is an intermediary host and vector of several parasite species, from protozoans to nematodes 1-3.
The nematodes of the Superfamily Metastrongyloidea, Order Strongylida, stand out among the typical parasitic load of A. fulica. During the adult stage these organisms are located in pulmonary arteries, in the left ventricle, in mesenteric veins, or in the pulmonary bronchia of carnivorous vertebrates 4. When they are at their first larval stage (L1) they pass through the gastrointestinal tract of the host and are expelled through feces. At this developmental stage the L1 larva uses gastropods as intermediary hosts, inside which it develops until it reaches the third stage (L3) and is ingested to go back to the vertebrate host 4-5. In other cases, paratenic hosts are also used, where the larva survives but does not develop.
Among the Strongylida nematode species that are found in A. fulica, the genera Angiostrongylus and Aelurostrongylus, are conspicuous because they cause diseases in animals and humans. Angiostrongylus vasorum, A. cantonensis, A. costaricensis, and Aelurostrongylus abstrusus have been the focus of attention as they can affect the health of domestic animals and human beings 4,6,7.
The rapid dispersal of A. fulica in tropical areas worldwide, added to its high potential as an intermediary host to Metastrongyloid nematodes has resulted in research on its parasitic prevalence focused on nematodes of the Angiostrongylus genus, of which the species A. cantonensis and A. costaricensis have caused hundreds of deaths through eosinophilic meningitis, which is endemic to Asia, and abdominal angiostrongyliasis, which is endemic to America, with the presence of A. fulica being associated in most cases 1,5,8. Molecular tools have recently begun to be used, such as antibodies, to diagnose diseases caused by Angiostrongylus9, and morphological and molecular tools based on PCR or alternatively LAMP have been usedin addition to identify helminth species 10,11.
For several years, A. cantonensis has been identified and associated with the African snail in several countries of Asia and Oceania. In America, this association has been demonstrated in the United States, Cuba, Jamaica, Bahamas, Puerto Rico, Dominican Republic, Haiti, Costa Rica, Brazil, Ecuador, Venezuela, and Argentina 5,12,13. Despite this, there is a lack of information on the real impact of this invasive mollusk on nematode dynamics, more so when there are no practical and effective diagnostic methods in countries where the snail plague is recent or the epidemiological and clinical impact of the nematodes, especially the genera Angiostrongylus, is unknown, as is the case in Colombia 5,14. Therefore, the objective of this study was to determine the presence and prevalence of Strongylida nematodes in Achatina fulica of the Valle del Cauca, Colombia, mainly of those nematodes potentially pathogenic for humans.
MATERIALS AND METHODS
Study area. Achatina fulica individuals were collected in nine cities of the Valle del Cauca Department, in western Colombia (Figure 1). Seven cities, Jamundí (3º15’N-76º32’’W),Cali (3º26’’N-76º31’’W), Palmira (3º31’N-76º81’W), Buga (3º54’07’ N-76º18’4’ W), Tuluá (4º05’N-76º12’W), Bugalagrande (4º12’N-76º18’’W) and Cartago (4º44’N-75º54’’W), were located along the geographic valley of the Cauca River, over a flat topography with an average elevation of 1000 m about mean sea level, 900 mm annual precipitation and average temperature of 25ºC. Another city, Dagua (3º39’N-76º41’’W),was located on western flat of the western Andes mountain at 1235 m about mean sea level, 1159 mm, annual precipitation and average temperature of 24ºC, and the last one city, Buenaventura (3º53’N -77º 05’W), the main maritime port of Colombia in the Pacific coast, was located within the biogeographic Chocó region, at 7 m about mean sea level, 7650 mm annual precipitation and annual temperature ranging between 25 and 28ºC 5.
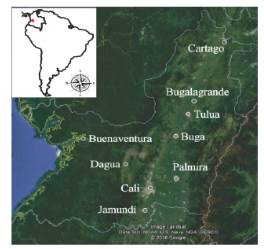
Figure 1 Sampling sites of Achatina fulica in the Valle del Cauca, Colombia.Landsat image center at 3°45’2.89”N - 76°32’52.17”O. Height 350 Km. Data from: SIO, NOAA, U.S. Navy, NGA, Gebsco. Source: Google Earth pro® 2016.
Animal collection. Taking into account the strong association of the giant African snail and the urban centers of the Valle del Cauca Department 5, the collection of individuals was focused inside the urban perimeter. Thirty cells (each of 2 km2) that encompassed the nine selected cities were defined, and three 16 m2 plots were established randomly within each cell. Animals were collected by hand within each plot and kept in individual ziploc bags for transport to the laboratory.
Parasitological analysis. To obtain A. fulica parasites, direct visual examination of snails larger than 50 mm was carried out. Specimens larger than 50 mm have greater probability of carrying nematode larvae 7,8. Individuals were first washed externally to avoid sample contamination by soil or other waste stuck to the shell. Shell length was measured using a micrometer (0.01 mm) and were weighed using a digital scale (0.1 g). Then were extracted mechanically from the shell and dissected to obtain lung tissue. This tissue was placed in a Petri dish with water along with the remaining organs, including the foot. The lung tissue was cut in 1 x 1 cm pieces and placed on microscope slides for observation under a stereoscope. The other organs were examined by compression between two 10 x 12 cm glass pieces. Nematodes were separated from the tissue or collected from the mucus. They were washed in saline solution and fixed in hot AFA solution, then mounted on a slide in reactive grade glycerine to clear them and allow the observation of structures of taxonomic value. Identification of nematodes to genus at the larval or cyst stage was based on Thiengo 15, Ash 16, Lv et al 17, Moreira et al 8 and Rebello et al 18.
Ethical aspects. The giant African snail has been declared an invasive exotic species and research permits are not required by the environmental authorities. Safety regulations dictated by the Ministry of Environment 19 for handling of A. fulica in Colombia were followed.
Statistical analysis. Three parasitological parameters were calculated in order to evaluate the epidemiological state of the A. fulica population regarding Metastrongyloid nematodes of public health interest. Prevalence was calculated as the number of hosts infected by a particular parasite species divided by the number of inspected hosts expressed as a percentage. The mean abundance was calculated as the total number of individuals of a particular parasite species divided by the total number of inspected hosts. Finally, the mean intensity was calculated as the total number of individuals of a particular parasite species divided by the number of hosts infected for that parasite species 20.
Prevalence of Angiostrongylus in Cali and Buenaventura was compared using a Chi squared test, assuming 2013 prevalence records like expected values and 2014 prevalence records like observed data. Yates correction was used given the discrete nature of the data. In order to evaluate the tendency of parasites aggregation in the hosts, a dispersion index (ID) and Morisita Index (Im) were calculated. Passage program V. 2.0.11.6. was used.
RESULTS
A total of 116 giant African snails were captured a checked for Strongylida parasites, ranging in length between 52 and 120 mm; 46 were captured in 2013 and 70 in 2014 (Figure 2). During 2013, 16 infected individuals were found while in 2014 only 7 infected individuals were found, with a general prevalence of 19.8% (Table 1). The cities with highest prevalence were Cartago (60% during 2013), followed by Buenaventura (42.9% 2013 and 30.0% during 2014) and Cali (33.3% during 2013 and 30.0% during 2014). (Table 1).

Figure 2 Giant African snail (Achatina fulica) individual collected for parasitological analysis in the Valle del Cauca, Colombia.
Table 1 Infection parameters of Metastrongyloid nematodes in giant African snails A. fulica during two seasons (2013-2014) in the Valle del Cauca, Colombia. n: specimens examined. I: specimens infected. TP: total parasites. P: prevalence.MA: mean abundance. MI: mean intensity.
| Season | Location | N | I | TP | P | MA | MI |
|---|---|---|---|---|---|---|---|
| 2013 | Cali | 27 | 9 | 32 | 33.3 | 1.2 | 4 |
| Buenaventura | 7 | 3 | 10 | 42.9 | 1.43 | 3.33 | |
| Cartago | 5 | 3 | 26 | 60 | 5.2 | 8.67 | |
| Dagua | 7 | 1 | 1 | 14.3 | 0.14 | 1 | |
| General | 46 | 16 | 69 | 34.8 | 1.5 | 4.31 | |
| 2014 | Buenaventura | 10 | 3 | 403 | 30.0 | 40.3 | 134.3 |
| Buga | 10 | 0 | 0 | 0 | 0 | 0 | |
| Bugalagrande | 10 | 0 | 0 | 0 | 0 | 0 | |
| Cali | 10 | 3 | 534 | 30 | 53.4 | 178.0 | |
| Jamundí | 10 | 1 | 23 | 10 | 2.3 | 23.0 | |
| Palmira | 10 | 0 | 0 | 0 | 0 | 0 | |
| Tuluá | 10 | 0 | 0 | 0 | 0 | 0 | |
| General | 70 | 7 | 960 | 10.0 | 13.7 | 137.1 | |
| 2013-2014 | Total | 116 | 23 | 1029 | 19.8 | 8.9 | 44.7 |
Most larvae were observed swimming freely in the mucus expelled by the snails or as cysts (Figure 3). Larvae were classified in three genera: Angiostrongylus (13.8% prevalence), Aelurostrongylus (2.6%), and Strongyluris (2.6%) (Figure 4, Table 2). The genus Angiostrongylus was recorded in five of the nine examined cities (Table 2), with the highest prevalence in the Cartago (60.0% during 2013), followed by Cali and Buenaventura (30% in both cities during 2014). Furthermore, the city with highest positive records of Aelurostrongylus was Buenaventura (28.6% during 2013), while Strongyluris was only recorded in Cali during 2013 with a prevalence of 11.1% (Table 2).
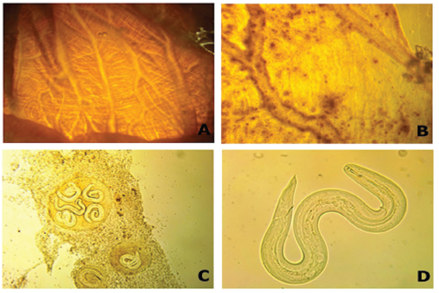
Figure 3 Results of the parasitological analyses carried out in Achatina fulica individuals in the Valle del Cauca, Colombia. A. Not parasitized lung tissue, B. Lung tissue infected with cysts, C. Types of cysts found, D. Nematode larvae of the Angiostrongylus.
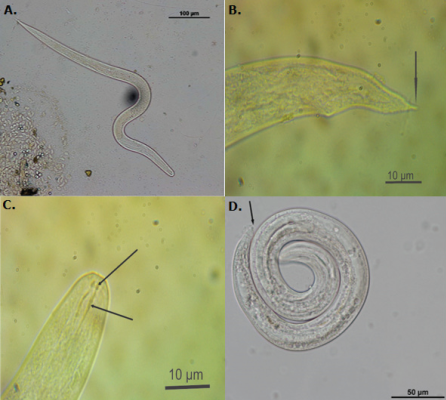
Figure 4 Larve of Metastrongyloid nematodes recorded in the giant African snail Achatina fulica in the Valle del Cauca, Colombia. A. Strongyluris, B. Tail of Angiostrongylus ssp. the narrow indicates the fine termination exclusive in the genera, C. Stylet structure from Angiostrongylus a character reported for the metastrongyloid larve, D. Aelurostrongylus larve, the narrow indicate
Table 2 Infection parameters of Metastrongyloid nematode species in giant African snail A. fulica individuals from five cities of the Valle del Cauca. n: specimens examined. I: specimens infected. TP: total parasites. P: prevalence. MA: mean abundance. MI: mean intensity (MA: mean abundance. MI: mean intensity.)
| Genera / Locality | n | I | TP | P | MA | MI |
|---|---|---|---|---|---|---|
| Angiostrongylus | 116 | 17 | 1010 | 14.7 | 8.7 | 59.4 |
| Cali 2013 | 27 | 5 | 20 | 18.5 | 0.7 | 4.0 |
| Buenaventura 2013 | 7 | 1 | 3 | 14.3 | 0.4 | 3.0 |
| Cartago 2013 | 5 | 3 | 26 | 60.0 | 5.2 | 8.7 |
| Dagua 2013 | 7 | 1 | 1 | 14.3 | 0.1 | 1.0 |
| Cali 2014 | 10 | 3 | 534 | 30.0 | 53.4 | 178.0 |
| Buenaventura 2014 | 10 | 3 | 403 | 30.0 | 40.3 | 134.3 |
| Jamundí 2014 | 10 | 1 | 23 | 10.0 | 2.3 | 23.0 |
| Aelurostrongylus | 116 | 3 | 14 | 2.6 | 0.1 | 4.7 |
| Cali 2013 | 27 | 1 | 7 | 3.7 | 0.3 | 7.0 |
| Buenaventura 2013 | 7 | 2 | 7 | 28.6 | 1.0 | 3.5 |
| Strongyluris | 116 | 3 | 5 | 2.6 | 0.1 | 1.7 |
| Cali 2013 | 27 | 3 | 5 | 11.1 | 0.7 | 1.7 |
A total of 1010 parasites of the genus Angiostrongylus were observed, including larvae and cysts of the lung tissue (Figure 3), with a general prevalence of 14.7% and mean abundance of 8.7 parasites/host. All Angiostrongylus individuals registered in a single individual from Jamundí were found swimming freely in the mucus of the lung cavity. In Buenaventura 7% of Angiostrongylus individuals where detected moving and 93% were cysts, whereas in Cali all individuals were cysts (Figure 3).
Total prevalence of Strongylida nematodes in A. fulica was significantly lower during 2014 compared with 2013 (X2 5.81, p 0.01). However, mean intensity was higher during 2014 compared with 2013 (Bootstrap 2000 repeat, t -4.13, p 0.01), and with the exception of Cartago, during both studied years, the Strongylida nematodes in A. fulica exhibited an aggregated distribution in all locations (Table 3).
DISCUSSION
All Strongylida nematode associated to A. fulica specimens off Valle del Cauca could also use native gastropods (e.g. Veronicillidaeor Strophochelidae) and vertebrates from the Orders Carnivora (especially Canis and Felis) and Rodentia (such Rattus) as intermediary hosts 8,13,19. Therefore, it is to be expected that African snails would interact with helminths present in the ecosystems they invade, although we should not rule out the possibility of introduction of parasites from the mollusk´s place of origin 14.
Regarding the genera Angiostrongylus and Aelurostrongylus for which there are records of zoonosis, the stablished parasitological parameters were similar to those recorded in Brazil and some countries of the Asian continent, where abundant cases of A. fulica containing these nematode genera have been reported 8,21.
In the Valle del Cauca, the presence of Strongylida nematode tended to be higher in the cities with influence from the Pacific region (e.g.Buenaventura, Dagua), probably due to the favorable environmental conditions that the biogeographic Chocó region offers, not only for hosts but also the parasites, as these are sensitive to environmental conditions both within and outside their hosts 5,21,22. Moreover, African snail also prefers sites such as garbage dumps, landfills and empty lots 15, where there is a higher probability of contact with rats, which are necessary for nematodes to complete their life cycle. These sites are common in the studied areas due to conditions of poverty in the human communities.
Prevalence about of 10% in the five cities where Angiostrongylus were detected (Cali, Jamundí, Cartago, Buenaventura, Dagua), is a clear indicator that the presence of the African snail can lead to public health problems in those locations, not only due to the high parasitic load recorded but also due the higher probability of people becoming exposed to direct contact with these animals 5,23.
Generally, the distribution of parasites tends to be aggregated in hosts 24. In the Valle del Cauca cities, the distribution of Strongylida nematode was aggregated, with exception of Cartago, where the highest prevalence was registered. Usually, the aggregated distribution of parasites in hosts is the results of the heterogeneity in the encounters rate between susceptible and infected hosts/vectors, variations on host physiological conditions or differences in habitat quality 25. Specifically, in Strongylida nematodes it has been demonstrated that the environmental conditions that regulate the distribution of intermediate hosts, modulate the encounter probability with the final host, affecting the production of the number of eggs and L1 larvae necessary for infection 22-26.
It should be noted that other Strongylida nematode which could affect the health of domestic and wild species 4, were recorded in African snails from the Valle del Cauca. These genera, Aelurostrongylus and Strongyluris, has not been reported in other gastropods or another studies for Valle del Cauca region. In conclusion, A. fulica is a mollusk that can potentiate the transmission rate of Strongylida nematode to wards humans in Valle del Cauca region, due to its high reproduction rate and human-mediated dispersal capacity 1, which favor nematodes dispersal and increasing the encounter rate. Although the African snail can be an important vector of transmission of these parasites, the role that native mollusk species could be playing at the local level in the life cycle of these parasites should not be underestimated.