Introduction
Solanum lycopersicum L. or tomato (Solanaceae) is one of the most important vegetable crops worldwide (Elkelish et al., 2020; Vásquez & Castaño, 2017; Yuqing et al., 2018). It is consumed both fresh and processed (Mahieddine et al., 2018), and its production has increased due to its high demand (Ahmad et al., 2017; Katirci et al., 2018). It is an economically important crop in various countries (Ebrahim & Saleem, 2017), placing its origin in the Andean region of Colombia, Chile, Peru, and Bolivia. However, there are indications that it was domesticated mainly in Mexico (Medina et al., 2017; Rahman et al., 2018). Furthermore, tomato is considered a healthy food due to its properties, attributed mainly to a component called lycopene (Navarro & Periago, 2016), characterized by its high antioxidant, vitamin, anti-inflammatory, and antiproliferative activities (Amjad et al., 2018; Chen et al., 2019). It is also associated with several health benefits, such as reducing the risk of chronic cardiovascular disease and cancer (Adekiya & Agbede, 2017; Johnson et al., 2019). Besides, it contains β-carotene, vitamins C and E (Elbadrawy & Sello, 2016; Park et al., 2018), and phenolic compounds (Tembe et al., 2018), such as flavonoids and polyphenols (Amin et al., 2018).
Seeds are the support of the agricultural industry (Zhang et al., 2018); therefore, generating information on seed quality is of great importance to ensure rapid germination and plant uniformity in the field (Faber et al., 2015), and for their conservation in germplasm banks (Al-Hammad & Al-Ammari, 2017). Due to the great importance of this parameter (seed quality), various methods have been developed, including the implementation of near-infrared spectroscopy (Shrestha et al., 2017). However, agriculture needs practical and feasible tests regarding time and resources, among which topographic staining with tetrazolium (TZ) stands out, as it is an economical, effective and rapid test (Lima et al., 2018) to determine the viability of any type of seed (Costa et al., 2018).
The use of rapid methods to know the viability is important to accelerate decision-making regarding the management of seed batches (Medeiros et al., 2015). This test is based on the activity of dehydrogenase enzymes that reduce TZ (2, 3, 5-triphenyl tetrazolium chloride) salt in living seed tissues, by generating diphenyl formazan, a non-diffusible red compound. This color compound indicates respiratory activity and viability of cells and tissues; on the contrary, dead tissues do not show any coloration (Campos & Kossmann, 2017; Cripa et al., 2014; Salazar et al., 2018; Salazar, Quintero, & Bustos, 2020). However, for the absorption of the TZ solution to be adequate and the test results to be conducive, some species require their seeds to be prepared before immersion in the TZ solution, a procedure known as preconditioning or pretreatment (Caravita & Takaki, 2014; Jácome & Dos Santos, 2010; Nascimento et al., 2016). There are various pretreatments, including hydration, which promotes the activation of the enzymatic system to obtain sharper staining (Leitzke et al., 2017). Likewise, when the seeds have hard layers, it is recommended to scarify them before staining, and, for this, sodium hypochlorite is usually used, as it is even more efficient than sulfuric acid (Vásquez et al., 2019).
Apart from the importance of employing a pretreatment, there are other parameters such as solution concentration, exposure time, and temperature, essential for adjusting the methodology adapted for each species (Gimenez et al., 2014; Pereira et al., 2017; Torres et al., 2018). Generally, the focus is to improve methodologies in order to utilize lower concentrations of the tetrazolium solution (Salazar, Quintero, & Moreno, 2020; Salazar, Quintero, & Rojas, 2020), thus, optimizing the use of the financial resources of the laboratory; in this way, a more extensive range of samples can be analyzed (Leitzke et al., 2017). According to the above, the aim of this study is to determine the efficacy of pretreatments for the optimization of the tetrazolium test in S. lycopersicum.
Materials and methods
Plant material
Ripe chonto tomato fruits of the variety Libertador were collected from crops established in the municipality of Cáchira (07°44'27"N; 73°02'55"W) at an altitude of 2,025 m a.s.l., in the department of Norte de Santander, Colombia. The seeds were removed from the respective fruits and dried at room temperature; subsequently, they were kept in kraft paper bags for 20 minutes in a controlled environment (20 °C and 55% RH), to avoid deterioration during the study (Santos et al., 2007). The research was carried out in the Biology laboratory of the Faculty of Basic Sciences, at Universidad Francisco de Paula Santander.
Seed pretreatment and viability
The following pretreatments were studied to optimize the effectiveness of the test and compare two of the most used compounds: immersion in distilled water with proven efficacy as pretreatment in soybeans (Pereira et al., 2019); Osmotic conditioning in the Solanaceae Physalis ixocarpa Brot. (Marín et al., 2007; Lallana & García, 2013); Sodium hypochlorite at 1.0 % with proven efficacy as chemical conditioning in Solanaceae and Brassicaceae (Valqui, 2017; Vásquez et al., 2019) for 10 minutes. Likewise, a control was used consisting of the non-application of a pretreatment (Benítez et al., 2013). For this, the syringe method described by Salazar (2012) was implemented as follows: a group of seeds was placed inside a 5 mL syringe equipped with a cloth filter (Salazar & Vega, 2017). At the end of the pretreatment period, three rinses with distilled water were carried out to eliminate the excess chlorine solution. Five replicates of 100 seeds were used, to which 5 mL of the TZ solution was added at three concentrations (0.25 %, 0.15 %, and 0.1 %), and three exposure times (6 h, 12 h, and 24 h), at a temperature of 25 °C under dark conditions. The viability of the seeds was classified with the help of a LEICA EZ4 stereoscope microscope, establishing their red coloration as a parameter.
Germination test
The viability data obtained was corroborated through a germination test (Espitia et al., 2017). Five repetitions of 100 seeds each were used; these were placed to germinate on paper towels moistened with distilled water in an amount equivalent to 2.5 times the mass of the dry substrate (Carvalho et al., 2013; Macedo et al., 2017; Oliveira et al., 2018), in a previously disinfected plastic container and under dark conditions for 72 hours (Salazar & Botello, 2018). Germinated seeds were those that showed a radicle of at least three millimeters long (Kaye et al., 2018), and it was expressed as germination percentage.
Statistical analysis
With the data obtained, an analysis of variance (ANOVA) was carried out to determine the effect of the treatments on the viable tomato seeds. The means were compared with Tukey's multiple DSH (Significant Honest Difference) range test, to establish if there are significant differences with the probability of p ≤ 0.05, with the help of the statistical software Statgrafic Centurion version XVI.
Results and discussion
Tetrazolium test
The viability percentage of the seeds was significantly affected by the exposure time (24 h in table 1), the concentration of the tetrazolium solution (0.25 % with means above the rest of the concentrations), and the applied pretreatments (the use of chlorine at 1 % was above the control and distilled water), corroborating in this way the importance of these parameters and how relevant it is to handle them to optimize the tetrazolium test. Pereira et al. (2019) highlight the importance of preparing the seed before exposure to the tetrazolium solution, since pretreating the seeds of Glycine Max L. (Fabaceae) with water resulted in a sharper coloration of the embryo, facilitating the classification. The exposure time and concentration is a determining parameter in the TZ test since, in the various tests carried out, the development of the test maintained a similar pattern, i.e., with a more extended period and concentration (0.15 % is higher, but without significant differences with 0.25 %), the intensity of the staining increases in living tissues (table 1). Therefore, the classification of viable and unviable seeds is facilitated (Lamarca & Barbedo, 2014).
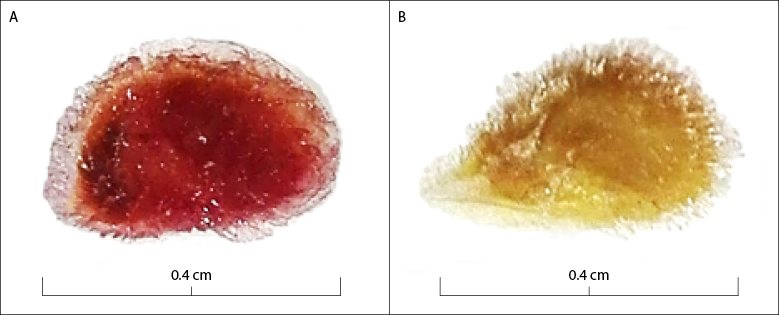
Source: Elaborated by the authors
Figure 1 Viability of Solanum lycopersicum seeds using the tetrazolium test. A. Viable seed; B. Nonviable seed
Table 1 Viability of Solanum lycopersicum seeds subjected to three pretreatments and evaluated by the tetrazolium test
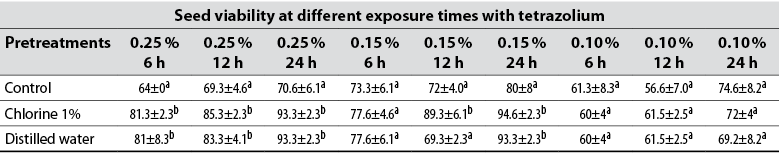
Note. Values with different letters in each column indicate statistically significant differences, according to Tukey’s HSD test (p ≤ 0.05).
Source: elaborated by the authors
However, the highest percentages of viability (94.6 % and 93.3 %) were obtained by implementing a concentration of 0.15 % during 24 hours, both in the pretreatment with sodium hypochlorite (NaClO) as well as with the one employing distilled water, respectively, being statistically homogeneous with each other (table 1). Thus, the importance of preparing the seeds before immersing them in the TZ solution is highlighted in relation to the control (Mendes et al., 2009), so that similar viability results were obtained without significant differences of the tomato seeds when using both the concentration of 0.25 % (70.6 ± 6a in the control, 93.3 ± 2.3b with the use of chlorine at 1 %, and 93.3 ± 2.3b with the use of distilled water) and in 0.15 % (80 ± 8a in the control pretreatment, 94.6 ± 2.3b with the use of chlorine, and 93.3 ± 2.3b in the distilled water pretreatment) with an exposure time of 24 hours (table 1).
These results are correlated with those obtained by Jácome and Dos Santos (2010), who recommends using a concentration of 0.15 % to evaluate the viability in seeds of Leucaena leucocephala (Lam.) De Wit. (Fabaceae). The appropriate methodologies must allow the good development of the test and vary depending on the evaluated species. For example, Lamarca and Barbedo (2014) studied three species of the genus Eugenia L. (Myrtaceae) that require different concentrations with the same exposure period for the good development of the viability test.
The lowest viability values were obtained when implementing the concentration of 0.10 % considering the coloration (figure 1), since the seeds were weakly stained, making it difficult to interpret the results regardless of pretreatment and time of exposure to the TZ solution; further, these were statistically homogeneous with each other (table 1). The appropriate methodology for the TZ test must facilitate the differentiation of viable from non-viable tissues, and be able to distinguish seed batches with different physiological quality (Santos et al., 2017). In this case, the seeds that did not express their coloration were considered unviable, when slight staining may be due to the low concentration of the solution utilized, so the use of the 0.10 % concentration to evaluate the viability in seeds of S. lycopersicum is not recommended.
Studies conducted by Ribeiro et al. (2010) in triticale seeds (x. Triticosecale Wittmack, Poaceae) indicate that the concentration of 0.1 % can be used to determine the germination capacity of the seeds; however, the identification of viable seeds is difficult due to the low tissue staining. It should be noted that the interpretation of the test results depends on an analyst who must meet certain parameters, such as knowing the parts of the seeds, as well as having experience and sufficient capacity to identify the seeds (Cripa et al., 2014).
Germination and viability comparison
The germination test allows identifying if the seeds did not germinate due to being latent, aborted, or if the embryo is damaged. Moreover, it is used to corroborate the viability results obtained with the TZ test (Salazar & Botello, 2018). The germination percentage of 96 % shows that the seeds had a high germination capacity. This value is directly related to the viability results obtained when using concentrations of 0.25 % and 0.15 % in a 24-hour period and exposing the seeds to immersion pre-treatments in sodium hypochlorite and distilled water for 10 minutes (table 1); these treatments are the most effective for evaluating the viability of S.lycopersicum seeds, not only due to the high correlation between the germination test and viability, but also because it allows a reduction of up to 0.15 % (reducing 0.10 % relative to 0.25 %) in the concentration of the TZ solution to be used without affecting the effectiveness of the test and the accuracy of the results. In this way, the application of laboratory resources is optimized, and a higher number of samples can be analyzed (Leitzke et al., 2017; Oliveira et al., 2018).
The highest seed viability values (94.6 %) and the highest correlation with the germination percentage (96%) were obtained when implementing the pretreatment with sodium hypochlorite. Generally, this strong oxidizing agent is widely used for its disinfectant characteristics. However, it has been used in orchid seeds to scarify them and stimulate their germination (Cripa et al., 2014; Salazar & Botello, 2008; Salazar, Botello, & Quintero, 2019; Salazar & Maldonado, 2020; Salazar, Quintero, & Bustos, 2020; Salazar, Torres, & Rojas, 2019). In various studies, this compound generally promotes germination through the degradation of the seed cover (Akbari et al., 2012) and accelerates the washing of the endogenous abscisic acid (ABA) of the seed (Bae et al., 2013, 2014).
Usually, seeds with hard layers need to be scarified, and, to carry out this process, NaClO is normally used to improve the effectiveness of the staining (Salazar & Vega, 2017). In seeds of Cypripedium lentiginosum P.J. Cribb & S.C. Chen (Orchidaceae) this compound is used to produce scarification of the seed cover, in such a way that its hydrophilic nature increases due to the oxidation of the cell wall (Jiang et al., 2017). The reduction of its hydrophobic characteristics allows a better contact of the tetrazolium solution with the seed tissues, hence, achieving optimal staining and facilitating the distinction between viable and non-viable seeds, increasing the reliability of the interpretation of the results.
Similarly, immersion in distilled water prior to staining offers a viable option to optimize the viability test. This agrees with the ones recorded by Lallana and García (2013), who obtained good viability results implementing immersion in distilled water in seeds of Trichocentrum jonesianum (Rchb. F.) M.W. Chase & N.H. Williams (Orchidaceae). The success of this pretreatment lies mainly in the activation of the dehydrogenase enzymes, responsible for releasing the hydrogen ions that reduce the tetrazolium salt to formazan, allowing adequate staining of the tissues, facilitating the classification of seed batches (Carvalho et al., 2014; Oliveira et al., 2018). Likewise, it was evidenced that it was not necessary to break the seed coat to obtain exact data on viability in relation to germination.
Carrying out pretreatment with sodium hypochlorite or distilled water using a 0.15% concentration of the solution and an exposure time of 24 hours in tomato seeds, allows generating information on the quality of the seed, revealing important aspects of a batch of seeds either for conservation or sowing (Duarte et al., 2017); besides, using high-quality seeds when sowing a crop ensures quick germination and emergence, as well as uniformity of the seedlings in the batch (Faber et al., 2015).
Conclusions
The use of distilled water and 1% sodium hypochlorite as pretreatment in Solanum lycopersicum seeds increases the efficiency of the tetrazolium test when using the concentrations of 0.25 % and 0.15 % for 24 hours. Likewise, the use of 0.10 % tetrazolium does not guarantee the efficacy of the test on tomato seeds.











 text in
text in 



