Introducción
Zinc is an essential element for natural plant growth and development, but its content in the soil is low compared to other essential nutrients (Sidhu et al., 1977). Zinc deficiency is widespread in many soils, especially those with a basic reaction (high pH) (Mam-Rasul, 2019). Calcareous soils, which are estimated at 10-30 % of the total soils in the world, suffer from zinc deficiency (Akrawi et al., 2021) because adsorption, precipitation, and fixation mechanisms create many forms of zinc (Akrawi et al., 2021), including free ions in the soil solution and soil reaction (Pérez-Novo et al., 2011), complex and fixed in the mineral composition of carbonate minerals (Al-Kaysi, 2000), silicate clays (Dahiya et al., 2005), phosphate-Fe oxides interactions, or adsorbed on the solid surfaces of the soil particles (Dandanmozd & Hosseinpur, 2010).
Quantity/Intensity (Q/I) is the ratio of the element amount in the solid phase to its concentration in the soil solution. The change in the Q/I curve reflects the soil’s capacity to resist change in the concentration of an element in the equilibrium solution (Beckett, 1964). Thermodynamic parameters are used to predict the final destination of an element in the soil system in the early stages of equilibrium; they are also considered when evaluating the change in the free energy related to the transport of an element from the soil solution to the sites near the double electrical layer. These are primary keys that help to understand the interactions or randomness associated with the equilibrium process (Adhikari & Singh, 2003). We used Woodruff’s (1955) equation to calculate free replacement energy based on the equilibrium constant values (ARZne) of the zinc ion’s activity ratios when there is no gain or loss to it. The negative result of the amount of energy change means that the interaction of zinc with soil is a spontaneous reaction and it not need heat, while a positive signal means that the reaction is non-spontaneous and needs heat-free energy, which is an excellent parameter to predict the amount of change in energy.
Several processes control the behavior of Zn Q/I parameters in soils, including adsorptiondesorption and precipitation-dissolution (Pasricha et al., 1987). Some physical and chemical properties of the soil affect the Zn adsorption process, such as the amount of clay, organic matter content, total active carbonate content (Osman et al., 1980), and increased soil pH. This research aims to study the behavior of zinc adsorption via Q/I parameters such as zinc potential buffering capacity, labile zinc, zinc activity ratio, Gapon Coefficient, and exchangeable zinc-free energy in order to evaluate zinc bioavailability in these soils using ten calcareous soils samples from Northern Iraq.
Materials and methods
Study area and sample collection
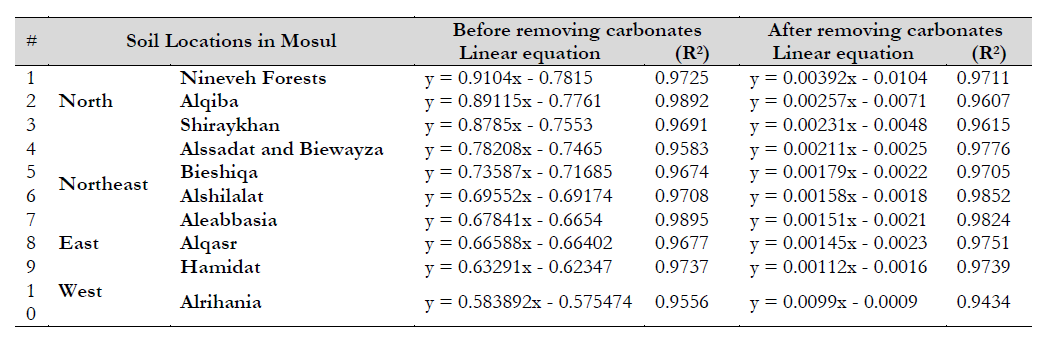
Ten samples of representative surface soil were collected from different locations in Mosul, Nineveh Governorate, Iraq (table 1; figure 1). Samples were dried, crushed, and passed through a 2 mm sieve for subsequent chemical and physical analyses Soils belong to Haplocalcids, Vertic Haplocalcids, according to Natural Resources Conservation Service and Agriculture Department (2010). Chemical and physical characteristics were determined based on soil analysis methods (Page et al., 1982). Organic matter was established by dichromate oxidation, and the cation exchange capacity was obtained by treating the soil with neutral 1N NH4OAC and 1N NH4OANa; final Na removal was read using a flame photometer. Electrical conductivity (EC) and soil pH were measured using a 1:2.5 soil to water suspension, while texture was determined according to Bouyoucos (1962). A t-test paired was used to compare texture and CEC before and after carbonate removal under the probability level 0.05, as shown in table 2 (Johnson & Bhattacharyya, 2019).
Table 1. The coordinates of the soil sampling locations in the study areas using GPS

Source: Elaborated by the authors
Carbonate removal of soil simples
Two hundred fifty grams of each soil sample was placed in a plastic container, and the removal process was carried out as follows:
1) Carbonate minerals were removed from the soil with 1N sodium acetate (CH3COONa.3H2O) at pH = 5 (Holford & Mattingly, 1975). This step was repeated three times until removing calcium carbonate.
2) The soil samples were washed three times with 0.5 N aqueous calcium chloride solution CaCl2.2H2O. This step was repeated three times with 0.05 N.
3) The soil samples were washed three times with 95 % ethyl alcohol to ensure all dissolved ions were removed, as confirmed by estimating the chloride ion concentration.
Experimental method and procedure
The quantity-intensity parameters of Zn+ were determined according to Salmon (1964) by adding 50 mL of 0.01M CaCl2 solution containing 0, 0.1, 0.2, 0.4, 0.6, 0.8, 1, 2, 5, 7, 10 mmol/L ZnCl2 to 2.5 gm of the soil sample before and after removing carbonates. The soil suspensions were shaken for 2 h and left overnight (24 h) for equilibration and then centrifuged. The suspensions of soil samples were filtered, and the supernatants were analyzed for Zn by atomic absorption photometer and Ca++ and Mg++ by titration with versinate (Pasricha et al., 1987). The Zn intensity (I) or activity ratio of Zn (ARZn) relative to Ca++ Mg++ species of each equilibrium solution was estimated. The change in the amount of Zn in solution gained or lost by the soil (±Δ Zn) was calculated by equation 1.
Where:
V = Volume of solution cm3
W = Weight of dry soil kg
ci = Concentration of added zinc
cf = Concentration of zinc after equilibrium (mg/L)
The Zn intensity factor in the liquid phase of soil is expressed as activity ratio AR Zn and computed from the measured Ca, Mg and Zn in the supernatant solution after equilibration. The activity ratio of Zinc (ARZn) was calculated according to the ratio law (equation 2) (Salmon, 1964)
Where:
ai = Ionic activity species (Ca, Mg, and Zn)
The ionic activity was calculated according to the Lewis equation (equation 3).
Where:
ai = Ionic activity
Ci = The species concentration of ions in mol/L
The ionic activity coefficients were calculated using the empirical Davies equation (equation 4).
Where:
yi = The mean activity coefficient of the electrolyte
Zi = Ion species valence
I = Ionic strength in mol/L
Where:
I = Ionic strength in mol/L
EC = dSm-1
From a plot of (±Δ Zn) versus the activity ratio, the Q/I parameters were obtained. The intercept of the Q/I curves on the ARZnequ axis, where Zn = 0, gave the soil Zn activity ratio at equilibrium (ARZn0), which means the soil solution Zn activity relative to the Ca + Mg at equilibrium. The (PBCZn0) was calculated as the slope of the linear section of the Q/I curve. Labile zinc (Δ Zn 0) was obtained from the intercept of the extrapolated linear part of the Q/I isotherm on the quantity axis. The free energy of Zn replenishment (-ΔGZnequ) was computed from equation 6, as reported by Salmon (1964).
Where R and T are gas constant and absolute temperature, respectively. Gapon constant was calculated according to equation 7.
Where:
kG = Gapon constant
PBCZn = Zinc potential of buffering capacity
CEC = Cation Exchange Capacity
Results and discussion
Soil properties
The solubility of all Zn minerals decreases 100-fold for each unit increase in pH. Soil pH has been identified in many studies as one of the main factors affecting zinc mobility and sorption in soils (Girija et al., 2013). The pH values for the studied soil ranged between 7.4 -7.8 (table 3). Zinc becomes more soluble as pH decreases; therefore, zinc is more mobile and increasingly available, whereas at pH > 7.7, the dominant form is Zn (OH)2 (Giordano & Mortvedt, 1980). Higher pH values form low solubility complexes with carbonate and hydroxyl ions (McGowen et al., 2001). EC values of the studied soils ranged between 0.92- 2.29 dS/m. These EC values o indicated non-saline soils because they were affected by the relatively fewer amounts of rainfall, which reduced the concentration of saline ions and are related to the low solubility of calcium carbonate. Generally, calcareous soils are not saline, as suggested by Holford and Mattingly (1975). The EC values of the studied soil locations were low and non-saline according to Al-Kaysi (2000).
The presence of carbonate minerals in calcareous soils will lead to coating around clay particles and organic matter, thus reducing the colloids’ surface area responsible for ion exchange and adsorption. Also, Zn sorption by CaCO3 and precipitation as Zn hydroxide or Zn hydroxyl carbonates control Zn solubility in calcareous soils (Papadopoulos & Rowell, 1989). The removal process will result in the detection of the covered surfaces and the emergence of new surfaces for exchange, thus increasing the adsorption capacity of the ion, which is consistent with our findings, as it increasedl the cation exchange capacitance after removing carbonates (figure 2).

Source: Elaborated by the authors
Figure 2. The cation exchange capacity values of the soils used in the study before and after removing carbonates
Carbonate minerals are one of the main components of the soil, especially in arid and semiarid regions. The removal of these minerals from the soil can impact some of their characteristics. The results of the conducted experiment showed that removing carbonate minerals from the soil changed some of their physical and chemical properties (table 3) (Sheikh, 2019). After removing the carbonate minerals from the soil, an apparent change in the soil’s texture appears before and after removing carbonate, as shown in Table 2. The percentage of clay also increased compared to the studied sites’ soils, while the proportion of silt and sand decreased in these locations, which is due to the role of carbonate minerals in linking minute clay with silt and sand, or the presence of clay in clusters size of silt and sand coated with carbonate minerals, making it one of the largest (figure 3). This result was confirmed by Osman et al. (1980), who found a change in the texture after removing the carbonate minerals from all study soils.

Source: Elaborated by the authors
Figure 3. Clay values in the soils under study before and after removing carbonate
Table 2. Physical and chemical characteristics before and after removing carbonates from the calcareous soils

Note *means significant difference between before and after removing carbonates for Texture and CEC Ns means non-significant difference between before and after removing carbonates for Sand
Source: Elaborated by the author
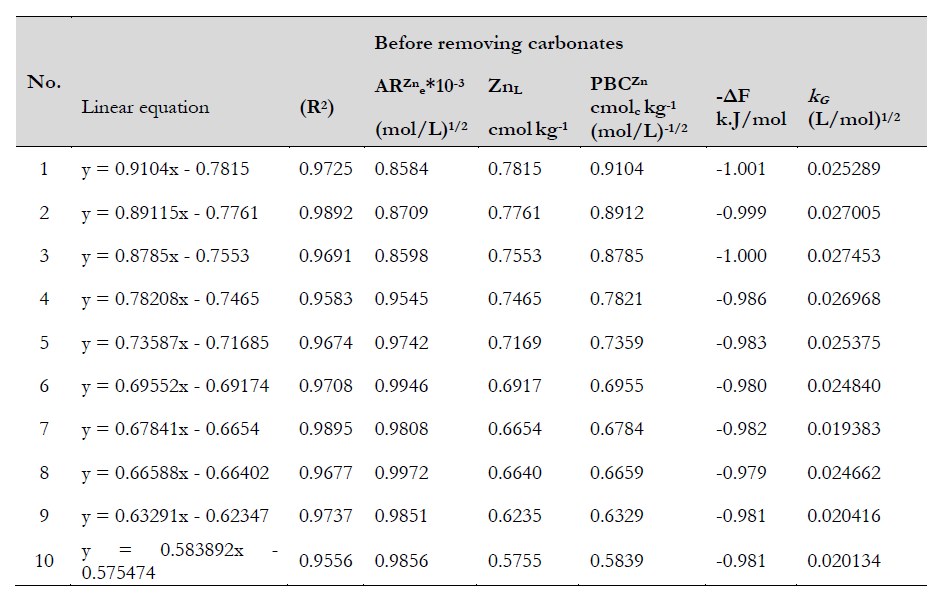
Table 3. Some quantity-intensity linear equations of zinc before and after removing carbonates
Note y= ΔZn= Adsorbed zinc (c.molc/kg)
x= ARZne
Source: Elaborated by the authors
Characteristics of linear regression equations for the Q/I relationship of zinc for the study soils before and after carbonate removal
The results shown in table 3 indicate the highly significant correlation of intensity and amplitude before and after removal, which is evident from the high R2 values, which ranged between 0.9556 and 0.9895 before removal and between 0.9434 and 0.9852 after removal. The equations shown in table 3 revealed the difference in slope of the linear relationship before removal, whose value (0.9104-0.583892) decreased (0.00392-0.0099) after removal. As for the cut-off, which expresses the moving zinc values, it was higher before removal than after removal, as it ranged from 0.575474 to 0.7815 before removal, and decreased (0.0009-0.0104) after removal.
Zinc activity ratio (ARZne) at equilibrium
The ARZne in soil solutions gave a reasonable estimate of zinc availability in soil. The differences in those soil parameters could be attributed to the difference in the Zn concentrations of equilibrating solutions, equilibration period, the Ca++ or Mg++ contents, and probably the differences in the soil minerals component. Q/I is defined as the ratio of the element amount in the solid phase to its concentration in the soil solution, while the change amount in Q/I reflects the soil’s buffering capacity. The latter is the resistance that the soil exhibits towards change in the element concentration in the equilibrium solution.
The intensity and amplitude parameters of the studied soil and their adsorption state before and after removing calcium carbonate are shown in tables 4 and 5. There are differences in the slope coefficient and intercept values, which is related to the different textures, mineral composition, carbonate minerals content, organic matter, and zinc forms. The difference is that the straight-line equation shows that the values of the straight-line inclination increase with carbonates from the soil. According to Pasricha et al. (1987), the straight-line inclination represents the soil’s buffering capacity.
The top of the linear curve allows estimating the zinc ion efficacy at equilibrium (ARZne) and the ability of the soil to regulate zinc ion depletion, as shown in tables 4 and 5. The values of ARZne ranged between 0.8584 and 0.9972×10-3 (mol/L)1/2 before removing carbonates and between 0.0909 and 2.7626×10-3 (mol/L)1/2 after removing carbonates, decrease which may be due to the increase in the proportions of fine clay in the soil particles. Values after removing carbonates shows the zinc ion’s preference for adsorption to the solid phase than for descending to the equilibrium solution, consistent with the results obtained for Gapon constant values.
As shown in tables 4 and 5, there is a reduction in Gapon values after removing carbonates. Also, there is a close relationship between the exchangeable soil capacity and the Gapon KG constant by Equation 8:
Potential buffering capacity (PBCZn)
The potential buffering capacity of zinc (PBCZn) is an indicator of the soil capacity to maintain a given zinc activity at equilibrium conditions against zinc losses by plant uptake or leaching. PBCZn is calculated from the slope of the linear part of the Q/I plot, a measure of soil ability to supply zinc activity in soil solution. So, it denotes the ability of the soil to replenish the exchangeable zinc. Before removing carbonates, the highest PBCZn value was 0.9104 cmol/kg (molL)−1/2 for soil site (1) and the lowest 0.5839 cmol/kg (mol/L)−1/2 for soil site (10), while the value after removing carbonates ranged between 0.00112 and 0.00990 cmol/kg (mol/L)−1/2 (table 4), which means an increase in the buffering capacity value. The buffering capacity of the soil under study increased due to the increase in carbonate content, which could be attributed to the carbonates factor’s effect on the zinc behavior in the soil. Moreover, the influence of pH and the precipitation of zinc as Zn (OH)2 reflects its release in soild and indicates an increase in the effort of the soil’s buffering capacity, which shows the zinc ion’s preference for adsorption to the solid phase than for descending into the equilibrium solution, similar to the results obtained by Pasricha et al. (1987) and Reyhanitabar (2012).
The labile pool of Zinc (ZnL)
It represents the amount of zinc capable of ion-exchange during equilibration between soil solids and soil solution. The labile zinc (ZnL) comprises two distinct components, namely nonspecifically held or readily available zinc (ΔZn0) and specifically held or hardly available zinc (Znx). The Q/I parameter values for studied soils before removing calcium carbonate are shown in table 4. The values of ZnL ranged between 0.5755 and 0.7815 cmol/kg, whereas the zinc values for studied soils ranged between 0.0009 and 0.0104 cmol/kg after removing calcium carbonate, which indicates that calcium carbonate plays a vital role in increasing zinc adsorption capacity via substitution and precipitation mechanisms. Thus, the increasing values of (ZnL) for natural soils are attributed to the high zinc affinity to the solid soil phase (table 4). Zinc behavior increases buffering capacity and ZnL. Besides high pH lets zinc be adsorbed as a monovalent cation such as Zn (OH3)- , Zn HCO3- , and Zn (OH)+ (Girija et al., 2013). In the same way, they remove calcium carbonate, reducing the exchangeable surface reaction of zinc with the solid phase.
Free exchange energies of Ca with Zinc (-∆F)
The standard free exchange energy (ΔF) expresses the potential of soil for zinc replenishment. It is an index of the amount of work needed to remove one mole of zinc from the exchange complex or fix it. It depends extensively on soil properties. This approach is used to characterize exchange equilibrium on clay and soil surface. The thermodynamic functions of soils have been used in recent years to describe zinc availability. Most of the thermodynamic studies on soils have involved calculating the free exchange energy from equilibrium. Also, thermodynamic parameters predict the final destination of elements in the soil system in the early stages of equilibrium. It is also considered to evaluate free energy change related to the transport of the element from the solution to the sites near the double electrical layer or clay equivalency layers. These primary keys help understand the interactions or randomness associated with the equilibrium process (Adhikari & Singh, 2003).
We used Woodruff (1955) to calculate the free replacement energy based on the equilibrium constant values. ARZne is the ionic activity ratios of the zinc ion when there is no gain or loss to it; the negative result of the amount of energy change means that the zinc’s interaction with the soil is a spontaneous reaction and does not need heat, while a positive signal means that the reaction is non-spontaneous and needs heat. Free energy is an excellent parameter to know the amount of change in energy. The -∆F values for studied soils before and after removing calcium carbonate are shown in tables 4 and 5. For natural soils, -∆F ranged between 1.001 and -0.979 kJ/mol, which is due to the negative turn of carbonates in covering clay minerals and prevents the occurrence of the specific adsorption process responsible for raising the free energy values of the adsorption process. Our results are consistent with Al-Othman et al. (2011) in that the low values of the substitution free energy indicate that the physical adsorption is the predominant mechanism in the adsorption process. After removing the calcium carbonate, -∆F was reduced and ranged between -1.319 and -0.841 kJ/mol because calcium carbonate removal exposed the specific execrable sides of clay mineral.
Gapon Selectivity Coefficient (kG)
The Gapon selectivity coefficient for Zn expresses the relative affinity soils may develop towards Zn in the presence of Ca and Mg both in the solid soil phase and soil solution under equilibrium conditions (Evangelou & Phillips, 1988). kG values fluctuated within the range 0.0193835-0.027453 (mol/L)1/2 before removing carbonates. Conversely, after the removal of carbonates, the values of the Gapon constant were 0.000045-0.00450 (mol/L)1/2. The changes in kG values are attributable to the levels of exchangeable Ca and Mg. The effectiveness ratio of clay surfaces is revealed after removing the carbonates and increases after washing, which resulted in a difference in the Gapon constant values (figures 2 and 3).
Conclusions
Zinc is an essential trace element for humans and plants at low concentrations, but at high concentrations, it may be toxic to plant, animals and cause various disorders for living organisms. Accumulation of heavy metals and movement in soils depends on several soil characteristics: soil pH, CEC, type of clay minerals, organic matter, and calcium carbonate content. This research studies zinc behaviors in ten calcareous soils to evaluate zinc translocation between solid and liquid phases via Q/I parameters. In the presence of carbonate, zinc was retained more tightly to form complexes compounds with relatively low solubility and became unavailable to plant uptake. Removing calcium carbonate reduces the values of the parameters ARZne, PBCZn , -∆F, ZnL, and kG, making zinc more bioavailable in the soil solution.