INTRODUCTION
Arca zebra is a pelecypod mollusk belonging to the superorder Filibranchia, family Arcidae.), It is distributed from the Gulf of Mexico and north of Florida to the north of Brazil (Lodeiros et al, 1999), but it is in Venezuela that they form commercially important banks (Prieto et al, 2001). The reproduction continues and the growth is conditioned by the enrichment and fertility of the waters, by the constant action of the trade winds that originate the coastal upwelling (Saint-Aubyn et al., 1999; Acosta et al, 2007).
Arca zebra is the second-most important product for small-scale fisheries in Venezuela and has a significant socioeconomic impact, mainly in the northeastern region (northern coast of the Araya Peninsula and in some communities of the islands of Margarita and Coche), with a production of 40,000 tons/year. The area with the greatest activity is Chacopata (11,214 tons and 2,313 fishing expeditions/month). It is estimated that 6,000 people benefit directly or indirectly from the extraction, processing and marketing of this product. The size of the mollusk ranges between 10 and 115 mm, with an average length of 56 mm, reproduction occurs continuously, reaching a peak between July and November, and mature specimens have been observed starting at 41 mm, with a lifespan of 2 years (Jiménez, 1998; 1999; Arias et al, 2002; Ramírez et al, 2006). The Ordinary Official Gazette No. 38,372, establishes as a quota of capture 200 sacks of 40 kg for boats that use harrows and 60 sacks for those that perform manual extraction (Casas et al, 2015). According to Peralta et al. (2016), each fishing expedition currently covers 27,830 m2 per vessel, harvesting, on average, 607 kg of A. zebra (without shells), with the entire fleet's catch approaching 30,000 kg.
Studies of A. zebra have focused on its growth (Prieto and Saint-Aubyn, 1998), specific production (Saint-Aubyn et al., 1999; Casas et al, 2015), condition indices (List et al., 2006; Villarroel et al., 2016), mobilization and use of their energetic substrates (Villarroel et al., 2016), aspects of fishing (Novoa et al., 1999; Ramírez et al., 2016), mollusk diversity (Prieto et al., 2001), associated benthic micro gastropods and macro mollusks (Narciso et al., 2005; Acosta et al., 2007; Licet et al., 2009) and the bycatch of gastropods associated with fishing (Nieves, 2012, Peralta, 2016). However, there is a gap in information about the organisms that live in association with A. zebra; therefore, this study identified, quantified and evaluated the negative effects fishing may have on the various taxonomic groups that make up A. zebra banks.
MATERIALS AND METHODS
Sample area
Samples were taken bimonthly from the fishing harvest of the Chacopata banks, located between Cabecera de Coche and Morro de Chacopata (latitude 10° 43'-10° 48' N and longitude 48'-63° 55' W; Figure 1). This area is approximately 70 to 80 square kilometers, with a depth that varies between four and nine fathoms. The samples were supplied by the ishermen, who harvest them in this area using trawl nets measuring 1.40 x 1.80 x 0.50 m, maneuvered from small wooden boats that are 10 meters long, 2.5 m wide and 0.5 m deep and powered by a 75-HP outboard motor. The net is dragged for 10 to 15 minutes at an approximate depth of four to eight meters. A. zebra, with all its associated fauna, is deposited in bags. This study analyzed one bag bimonthly from May 2010 to August 2011.
Processing of the samples
All the accompanying fauna was separated manually, with the help of tweezers and needles that facilitated the detachment of the organisms, mainly from the shells of A. zebra the mollusks and tunicates were placed in a 10% formalin solution. The polychaetes, crustaceans and echinoderms were preserved with 70% isopropyl alcohol to prevent decomposition, acidification and loss of some of their essential anatomical structures that are crucial to taxonomic studies.
Identification and classification for each taxonomic group
The biological material was examined under a stereomicroscope (Motic) and identified through the following taxonomic keys for mollusks: Morris (1960), Warmke and Abbot, (1962), Lodeiros et al.,. (1999), Macsotay and Campos (2001); crustaceans: Davant (1963) and Rodriguez (1980); echinoderms: Hendler et al. (1995) and Gomez and Hernandez (2011); polychaetes: Uebelacker and Johnson (1984), Linero-Arana (1999, 2011), and Fauchald (2003); and tunicates: Montes (1985). . Once the organisms were separated by taxonomic groups, they were counted and identified as far as possible up to the category of species. The taxonomic status of the species was verified using the World Register of Marine Species (WORMS) database (http://www.marinespecies.org/).
Calculation of the biomass
The dry biomass of each group was determined by drying the material in a VWR oven for a period of 48 to 72 hours at 60°C until a constant mass was obtained. The total dry weight of each organism was obtained using an Adventurer-pro electronic scale (Ohaus) with 0.001-g precision. To standardize the bimonthly biomass, the biomass was divided by the drag time (10 min) in relation to the area dragged and expressed in g/m2/hour of drag time.
Ecological indices
The structure of the community of organisms associated with fishing A. zebra was described in terms of abundance, richness of species, Sanders diversity (1968), equity (Pielou, 1969) and dominance (Krebs, 1985). These indices were calculated using the PAST statistical software. The result was obtained using the formula defined by Krebs (1985), which establishes three categories: (C) = constant: species present in more than 50% of the samples; (A) = accessory: species present in between 25% and 50% of the samples; and (a) = accidental: species present in less than 25% of the samples.
ABC Curves
To determine the degree of disruption of the macroinvertebrate community caught with bottom trawling, the Abundance / Biomass Comparison (ABC) method was used, which simultaneously compares dominance in terms of abundance and in terms of biomass (Warwick et al., 1987; Yemane et al., 2005). In a normal state, the biomass curve is above the abundance curve; in disturbed environments, the biomass curve is below the abundance curve. W, which represents the area of difference between the two curves, is obtained as follows: W=, where S describes the change in the total number of species, A is the total abundance in each sample i and B is the total biomass in each sample i. A negative value indicates that the biomass curve is below the abundance curve and suggests a community that has been disturbed (Warwick and Clarke, 2001). In this study, abundance was expressed in terms of CPUE (individuals per hour) and biomass was expressed in CPUE (kilograms per hour of trawling).
Statistical analysis
The different variables studied did not fit a normal distribution after different methods of homogenization were attempted to compare values for abundance, biomass, diversity and richness over the months of sampling, a nonparametric Kruskal-Wallis test was used (Siegel, 1994).
RESULTS
A total of 3.249 organisms were collected and 130 species were identified (Table 1), distributed over five Phylla: Mollusca (22 bivalves and 49 gastropods) with 71 species (54.96%), Annelida (Class Polychaeta) with 30 species (22.90%), Arthropoda (Class Malacostracea) with 15 species (11.45%), Echinodermata (1 Asteroidea, 4 Echinoidea and 6 Ophiuridea) with 11 species (8.40%) and Chordata (Class Ascidiacea) with 3 species (2.29%).
Table 1 Taxonomic classification of the organisms associated with the Arca zebra bank.

* First record for this area
** Microgastropods
*** First record for Venezuela
Percentage of contribution by family
The families with the highest percentages of contribution were Pteriidae (16.17%) and Eunicidae (13.01%). The remaining families contributed less than 10%: Serpulidae (9.31%), Mytilidae (8.40%), Crepidulidae (6.43%), Corallanidae (4.76%), Arcidae (3.88%), Mithracidae (3.58%), Xanthidae (2.70%), Pectinidae (2.70%), Chamidae (2.64%), Trochidae (2.55%), Muricidae (2.27%), Ophiothrichidae (1.97%) and Ascidiidae (1.76%).
Total abundance of taxa
Mollusks showed the greatest abundance, followed by polychaetes, crustaceans, echinoderms and ascidians (Figure 2). Although bimonthly differences (KW; P=0.62) were not measured between December and April, the greatest abundances were recorded, represented mainly by the mollusks P. imbricata and C. auricula and the annelids S. dendropoma and E. rubra. The remaining taxa showed less abundance, except for the crustaceans, represented by E. antillensis, which showed the largest number of organisms in November and December (Figure 3).

Figure 3 Bimonthly variations in the abundance of the major taxa associated with the Arca zebra bank.
Quantification of the biomass of different taxonomic groups
Mollusks contributed 81% of the total biomass, followed by echinoderms (17%), while crustaceans and ascidiae represented 2% (Figure 4). The species that contributed the highest biomasses were: P. imbricata, P. pomun, M. brevifrons and C. macerophylla, while Clypeaster sp. and Encope sp. were found among the echinoderms. Of the remaining groups, P. nigra, M. forceps and E. rubra accounted for the greatest biomass.
The bimonthly averages of the total biomass varied between 14.26 and 143.42 g/m2/hour trawl, with minimums in September and November, showing a tendency to increase in December, reaching the maximum value in August. The high biomasses were represented by mollusks and echinoderms, in all the sampling months. In July, December and August, the mollusks had the highest number of organisms, while, the echinoderms were in February, April and Augus, the mollusks, P. imbricata, contributed the largest biomass and in the echinoderms, Clypeaster sp. and Encope sp. (Figure 5).
Ecological parameters
The total richness did not show bimonthly variation (KW, P= 0.83), with the highest values occurring in February and April 2011 and the lowest in November 2010 and July 2011. Mollusks accounted for the largest number of species, especially in February 2011, while polychaetes showed the greatest richness in April 2011 (Figure 6).

Figure 6 Bimonthly variation of the total biomass and of the main taxa (g/m2/trawl hour) associated with the Arca zebra bank
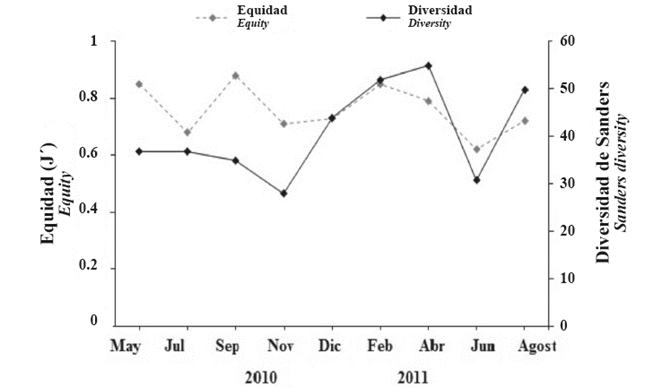
The total Sanders diversity measured in the area was 129.9. Mollusks showed the greatest diversity, followed by polychaetes, while echinoderms, crustaceans and ascidians showed the lowest diversity. The total equity indicates that the aggregations of bivalves are equitably distributed, while crustaceans and polychaetes were less evenly distributed than echinoderms and ascidians (Figure 7).
Bimonthly diversity ranged from 27 to 55 species, with peaks in April and February, while the lowest value occurred in November 2010. No bimonthly variations were observed (KW, P= 0.76; Figure 8).
Constancy
Of all the organisms identified (130 species), 22.14% were constant, while 19.08% were accessory species and 58.78% were considered accidental species. With regard to bimonthly constancy, only five species were found in 100% of the sample, namely M. forceps, P. nigra, E. lucunter, E. rubra and P. imbricata. The main accessory species were P. gibbosa, A. floridana, C. waltonense, Clypeaster sp. and Ophiocoma sp. The accidental species consisted of C. glauca, O. algicola and P. interruptolineata (Table 2).
Assessment of the impact of fishing activity n A. zebra
The Abundance / Biomass Comparison Curve showed that the total abundance of organisms was greater than the total biomass (Figure 9), suggesting that fishing of A. zebra has a negative impact on the associated organisms (W=-0.29). To estimate more accurately the variations of -W, curves were calculated for each taxon so that the groups that have been most affected could be determined. The echinoderms and crustaceans showed similar curves, in which abundance was greater than biomass; over time, the abundance showed a tendency to surpass the biomass, which is interpreted as a high degree of disturbance (W=-0.75; W=-0.53, respectively), with the echinoderms suffered greater stress. The mollusks were the least affected (W=-0.23). The polychaetes and ascidians showed very similar curves, reflecting a moderate degree of disturbance (W= 0.01 for polychaetes and W= 0.05 for ascidians) because the -W values were very close to zero.
DISCUSSION
A total of 3,249 individuals were counted. These values were higher than those obtained by Narciso et al. (2005) and Acosta et al. (2007) with 378 and 713 organisms respectively. It is important to note that these works focused on quantifying and identifying the bivalves and gastropods associated with the A. zebra fishery, while in this study all the fauna from the fishing activity was counted and identified; where the main taxonomic group was also constituted by the bivalves, represented mainly by P. imbricata. The abundance and biomass of this species is explained by the presence of small population aggregates, located in nearby areas could be constantly contributing individuals to the bank of Arca zebra. This corroborates the points made by Hernández et al. (2013), who indicate that the aggregations of A. zebra and P. imbricata, work as alternative habitats, usually sharing the same groups of organisms. The continuous reproduction of P. imbricata, according to Marcano et al. (2005) occurs throughout the year in the area, would explain the high incidence of individuals with sizes less than 30 mm. The reproductive status of P. imbricata showed that 70% of the total catch was in an indeterminate and immature state and in a lower percentage, organisms in mature and spawning state were observed (personal observation), confirming that the fishing gear used for the extraction of A. zebra is not selective, since it catches a high number of individuals that have not yet reached their first sexual maturity, which could be affecting the population renewal of P. imbricata, species that has a commercial importance and supports an important fishery in the northeastern Venezuela.
The number and composition of species associated with A. zebra in this study, explain that this arid acts a bioengineering species, generating a structural complexity with different niches that can be occupied by various taxonomic groups. Several studies point to the role of aggregations of bivalves as a potential habitat or as a refuge for benthic fauna (Eggleston 1998, Posey et al., 1999). The highest biomass values were contributed by P. imbricata and the gastropods P. pomun and M. brevifrons. In P. imbricata, it was related to the high abundance registered in all the sampling months, however, in the gastropods, this parameter was associated with the size of the organisms. Within the echinoderms, Clypeaster sp. and Encope sp., were those who contributed more biomass within their group, due to the size of their individuals, which ranged between 10 26 cm. The physical structure of their bodies, constituted by calcareous plates, also gives them a greater weight. The lowest values were represented by the polychaetes, related to the size and anatomical structure of their bodies. The magnitude of the biomass captured was related to the drag time, the type of fishing gear used, the fishing site and the environmental conditions of the area.
The second taxon with greater abundance and biomass, were the polychaetes, represented by Leodice rubra, Spirobranchus tetraceros and Oenone fulgida, the latter species, constitutes new registry for Venezuela. The bank of A. zebra not only has importance as an economic resource, it also constitutes an important substrate for the establishment of an important polychino-fauna in the area. The abundance of this group can be explained since the surface of the valves of this arid serve as a seat for many tubular species and also provide a greater availability of microhabitats for volatile species. The biomass and diversity recorded in this study suggest that this taxonomic group has important ecological niches in the bank, but could be harmed over time by the commercial exploitation of A. zebra.
The increases in biomass in most of the taxonomic groups coincided with the periods of coastal upwelling, which is associated with the recruitment pattern of many organisms in northeastern Venezuela; On the other hand, the quality and quantity of available food, is what explains the continuous renewal of the populations of A. zebra and together with it, the high diversity of the organisms that are associated with it. The changes in temperature during coastal upwelling also play an important role in the abundance and biomass of organisms, because many start reproduction processes based on variations in this environmental parameter. Five species with 100% constancy were observed, including several that are ecologically important, such as M. forceps, which contributes abundant larvae to the zooplankton (Hernández-Reyes et al., 2001). E. rubra makes possible the renewal of the area's organic matter (Rivera and Romero, 2002), occupying several levels in the food chain and acting as both prey and predator. The clusters of P. imbricata serve as a substrate for the colonization of other organisms (Hernández-Ávila et al, 2013). E. lucunter is important to carbon recycling, and its extraction can negatively affect these ecological processes (De Entrambasaguas, 2008). The high degree of constancy of these organisms is related to their reproductive activity and the continuous supply of nutrients the area receives from the coastal upwelling. The high percentages of accidental species (58.78%) suggests that clusters of A. zebra provide an important substrate or shelter for the establishment of a wide variety of organisms. The accidental species may indicate the existence of new organisms in the bank that until now have not been described for the area, such as the micro-gastropod Triphora melanura.
The Abundance/Biomass Comparison Curves showed that the groups most affected by the fishing of A. zebra were echinoderms, crustaceans and mollusks because the values for these three groups were negative, meaning their abundance curves were above their biomass curves. Echinoderms were the taxon most affected locally, with a low diversity, probably due to the death of these organisms caused by the pressure exerted by the A. zebra shells during trawling. The low degree of disturbance of polychaetes and ascidians is associated with the continuous movement and re-suspension of the bottom resulting from fishing. Among the crustaceans, scavenger species were most abundant, namely M. forceps, E. antillensis, and P. herbstii, possibly because these species could be replacing the most affected crustacean populations.
Both the bycatch and the target species are affected by the non-selective nature of the fishing gear used, and this changes the structure of the community, considering that the A. zebra bank provides an important substrate for the establishment and development of various populations of benthic organisms, many of which are commercially important. For this reason, there is a need to improve the pattern of utilization of this important fishery resource by modifying the trawling nets, increasing the mesh size to avoid catching both small organisms, such as certain sedentary species, and average-sized mobile species. This would minimize the impact of fishing on the fauna associated with natural banks of A. zebra.











 text in
text in