INTRODUCTION
Coral mortality over the last decades on Caribbean reefs was accompanied by an increase in the abundance of encrusting excavating sponges, which can directly kill coral tissue while simultaneously weakening coral skeletons (Rützler, 2002; López-Victoria and Zea, 2005; Ward-Paige et al., 2005; Schonberg and Ortiz, 2008; Chaves-Fonnegra and Zea, 2011). Cliona tenuis (Zea and Weil, 2003) (Porifera, Demospongiae, Clionaida, Clionaidae) is a dark brown encrusting and excavating, zooxanthellae-bearing sponge, which experienced a significant population increase following the massive die-off of the elkhorn coral, Acropora palmata (Lamarck, 1816), that occurred in the early 1980s across the Caribbean on exposed windward reef zones. The release of space from A. palmata mortality led to the opportunistic occupation by this sponge, which increased in abundance thereafter (Cortés et al., 1984; Williams et al., 1999; Aronson et al., 2002; Rützler, 2002; López-Victoria and Zea, 2005). Larvae of C. tenuis seem to preferentially settle on recently dead coral portions, grow laterally through pioneer excavating filaments that penetrate the skeleton underneath the polyps, and subsequently kill affected coral tissues and partly bioerode the coral skeleton (López-Victoria and Zea, 2005). Thus, C. tenuis can effectively displace and replace live coral cover (López-Victoria et al., 2003, 2006; López-Victoria and Zea, 2005). In addition, adult C. tenuis colonizing A. palmata branches can invade new live massive corals when loose branches are smashed against them during heavy swell, dispersing the sponge throughout the reef and eliciting further coral mortality.
Cliona tenuis can also invade from below and overgrow new coral colonies that have established on the sponge-colonized A. palmata branches (López-Victoria and Zea, 2004). However, the population boom of C. tenuis seems to have reached a standstill by the 2000 and throughout the 2010, as its ability to occupy new space at the expense of corals is being compensated by sponge tissue shrinkage, coral escape by upward growth (Marulanda-Gómez et al., 2017), and by macroalgal competition (González-Rivero et al., 2016).
Cliona tenuis is able to survive fragmentation and transplants can reattach and grow in new substratum (López-Victoria et al., 2003; López-Victoria and Zea, 2005). Strong surge from storms and hurricanes are progressively breaking dead fragments of A. palmata previously colonized by C. tenuis and thus likely increasing C. tenuis translocation throughout the reef (Marulanda-Gómez et al., 2017). As the frequency and intensity of tropical storms and hurricanes is expected to increase with climate change (Knutson and Tuleya, 2004), we hypothesized that this phenomenon of sponge dispersion and colonization of new massive corals via coral fragments will increase with time (López-Victoria and Zea, 2004). To test this prediction, we compared the frequency of massive coral colonization by C. tenuis through dispersion of dead branches of A. palmata and through other modes (i.e., from below), between 2002 and 2014, in the same reef sector at Islas del Rosario, Colombia.
MATERIALS AND METHODS
In a previous study carried out in 2002, López-Victoria and Zea (2004) determined the proportion of different modes of colonization of Cliona tenuis in the northern sector of the coral reef of the Pajarales complex at Majayura Island, Islas del Rosario, Colombia. In order to detect changes at the study site over the ensuing 12-year period, in July 2014 we repeated the measurements at the same site following the same methodology. A 560 m2 area of the reef was surveyed through successively deployed belt transects (20 x 4 m each) using an extended measure tape as a guide. Individuals of C. tenuis observed within two meters of each side of the tape were counted, using a 1 m rod as a reference, and their apparent modes of colonization were recorded. The same four modes of sponge colonization that were defined by López-Victoria and Zea (2004) were used: (D) direct contact, when an adult sponge-carrying A. palmata fragment was found still leaning against the coral, and the sponge had apparently colonized and spread from the contact point; (P) previous possible contact, when there was a sponge-carrying A. palmata fragment lying close (usually at or near the base), but not currently touching the coral on which the sponge was living; (U) unknown, when the sponge-colonized coral colony did not have any sponge-carrying A. palmata fragment lying close by; and (S) from the substratum, when a coral colony that had settled on an A. palmata dead branch had been reached and colonized by the spread of an adult sponge already present on the branch. Case (U) colonization was probably occurring from direct larval settlement, but there was no way to confirm it. Changes in the proportion of these four modes of colonization of C. tenuis from 2002 to 2014 were compared by a Pearson's Chi-squared test of independence, with the null hypothesis that the proportions of colonization modes were not different between years, using the R chisq. Test (frec) routine (R Core Team, 2016). Cover of corals and other major benthic components, which had been estimated in 2001 (López-Victoria and Zea, 2005), were estimated again in 2014. Percent areal cover was estimated from the measure tape guides deployed for the band transects (n=3 for 2001 and n=7 for 2014), by using the linear distance of the tape that intercepted each component, and calculated as percent length relative to the total tape length. Cover of each benthic component was compared between years by Mann-Whitney U tests using the R wilcox. test routine (R Core Team, 2016).
The colonization progress of four C. tenuis-colonized massive coral colonies that were marked between 2001 and 2002 (López-Victoria et al., 2003) and in 2004 (Márquez et al., 2006; Márquez and Zea, 2012), was also monitored from photographic series and field observations. These coral colonies had, when first marked, a leaning branch of sponge-carrying A. palmata from which C. tenuis had begun the colonization of the coral host. These coral colonies had been used by López-Victoria and Zea (2004) to calculate an approximate date of contact, and to relate it with major hurricanes or storms. In 2014, we again localized these coral colonies to evaluate the ability of the sponge to survive in the A. palmata fragment, and to spread into the massive host coral.
RESULTS
The number of live coral colonies colonized by Cliona tenuis more than doubled from 202 in 2002 to 423 in 2014. In 2014, 5.7 % of the sponge-coral interactions accounted for colonization of the coral through direct contact (D), and 4.0 % consisted of previous possible contact (P) (Figure 1). The A. palmata dead branches were large (> 1 m in length), and partially or completely covered by C. tenuis. In 4.7 % of the cases, the sponges had colonized the corals from the substratum (S), while in 85.6 % of the cases the origin of the colonization by the sponges was unknown (U). In 2014 we observed 24 new sponge-coral interactions in which C. tenuis had colonized the massive corals from a sponge-colonized A. palmata branch. We assumed these were new interactions because: i) between 2001 and 2004 we meticulously searched for (and marked) all coral colonies affected by C. tenuis in the core of the study site where we found the new cases; and ii) the distance advanced by the sponge into the coral was short enough not to have occurred before 2001-2002. Overall, the proportion of the different modes of colonization changed significantly from 2002 to 2014 (Pearson's Chi-squared test of independence X = 87.016, df = 3, p < 2.2x10-16). All modes of colonization from adult sponges showed a reduction from a half to almost a fifth in 2014 compared to 2002, while the unknown (U) cases almost doubled in 2014, although still being the more widespread case (Figure 1).

Figure 1 Percentage of Cliona tenuis individuals found colonizing coral colonies from the four modes of colonization defined in this work for 2002 and 2014. In both years, 560 m2 were surveyed; band transects were deployed in the same sector of the reef.
In 2014, we found C. tenuis colonizing ten coral species through direct contact (D) or from the substratum (S). Most colonization through direct contact (D) occurred on large, massive-growing species like Pseudodiploria strigosa (Dana), orbicella annularis (Ellis and Solander) and Siderastrea siderea (Ellis and Solander) (Table 1). Laminar, encrusting, low mound, branching or foliose coral species like Undaria agaricites (Linnaeus), Millepora alcicornis (Linnaeus), and Porites astreoides (Lamarck) were colonized mainly from the substratum (S). Colonization through direct contact (D) showed a reduction from 2002 to 2014 for most coral species. P. astreoides was not found to be colonized through direct contact (D) in 2014, but it continues to be the coral species more affected by the attack of C. tenuis from the substratum (S). Other differences between years include the colonization of Colpophyllia natans (Houttuyn) and the colonization from the substratum (S) in Pseudodiploria spp. and Diploria labyrinthiformis (Linnaeus), events that were not observed in 2002. The decrease of sponge colonization through direct contact is unlikely related to a reduction on coral cover between the studied years since no significant differences in mean percent cover of the major benthic components was observed (Mann-Whitney U-test, P > 0.05, Figure 2).
Table 1 Number and percentage of coral colonies overgrowth by Cliona tenuis by direct contact (D) or from the substratum (S) for 2002 and 2014. Data of 2002 from López-Victoria and Zea (2004).


Figure 2 Mean percent cover of major benthic components and Cliona tenuis in the studied area in 2001 and 2014. Values are means (± 1 SE). cro: coral rock (dead coral and pavement); lco: live coral; snd: sand and rubble; other: macroalgae and other invertebrates; cten: C. tenuis. Data for 2001 from López-Victoria and Zea (2005). Data obtained from 20 m linear transects deployed in the same sector of the reef, using the guide measure tapes of the band transects; n=3 for 2001, n=7 for 2014.
The marked colonies in which C. tenuis colonization originated through direct contact (D) revealed both advance and retreat of the sponge over time. The photographs series showed that the sponge increased its surface area during the first four years, but in the forthcoming years, some of the sponges started to show tissue loss (Figure 3) while others continued growing and undermining the coral host until the colony died almost completely. There was a particular colony of P. strigosa (colony ID 1913, Figure 3) that in 2002 had a large (> 1 m in length) branch of A. palmata leaning against it, from which the sponge had colonized the coral, but the coral had partly escaped by upward dome-like growth. In 2014, the A. palmata branch was found detached from the massive coral and C. tenuis had completely overtaken the space previously shadowed by the A. palmata fragment. This case confirmed the sequence of the process of translocation of a sponge-carrying A. palmata branch, colonization and spread of C. tenuis over a massive coral colony, coral partial escape, and detachment of the sponge-carrying branch (Figure 3).
DISCUSSION
Our predicted increase of adult C. tenuis dispersion via sponge-carrying A. palmata fragments was not supported by the data. Instead, the cases of colonization by this method during the intervening 12 years of our study (i.e., 2002 to 2014) decreased by one-half to one-third. However, the method of dispersion and colonization through these fragments is still occurring in the surveyed coral reef. Observations of newly colonized corals through translocation of A. palmata branches provides evidence for this phenomenon. Monitoring of previously marked cases of direct contact (D) confirmed how the process of colonization through translocated coral branches operates, from branch translocation, to sponge colonization and advance, to eventual coral escape (or death), and possible branch detachment.
The normal surge of the wind-exposed study site is by itself not strong enough to move the A. palmata branches. Thus, the translocation of sponge-carrying A. palmata fragments is likely mediated by heavy surge. In fact, initial time of C. tenuis colonization on massive corals, traced back in 2002 from sponge growth rates, showed to coincide approximately with hurricane swell reaching the surveyed coral reef (López-Victoria and Zea, 2004). With our finding of new cases in 2014, we confirm the idea that this mode of coral colonization by C. tenuis in Caribbean reefs through heavy surge is still occurring. The observed decrease for this mode of colonization from 2002 to 2014 is, however, contradictory, unless the same persistent process of branch translocation is taken into account.
Indeed, we showed that heavy surge can also remove a branch leaning on an invaded coral head, erasing the evidence of previous direct contact and invasion. In fact, four hurricanes and three tropical storms passed close to the studied area from 2002 to 2014 (Table 2) providing a scenario for both further colonization and erasure of evidence to take place. The concomitant absolute and relative increase from 2002 to 2014 of cases with unknown mode of colonization (i.e., part of which may have had the evidence of direct contact erased) further supports this hypothesis.
Table 2 Hurricanes and tropical storms that affected the study area between 2002 and 2014. Tropical storm: maximum sustained winds 34-63 kt (17-32 ms-1). Hurricane: maximum sustained winds 64 kt (33 ms-1) or higher. Major hurricane: maximum sustained winds 96 kt (50 ms-1) or higher. Dates based on UTC time and include tropical depression stage.
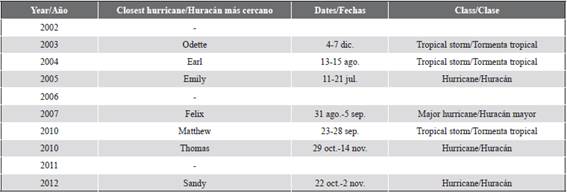
Source: Hurricane Research Division of AOML/NOAA (https://www.aoml.noaa.gov/hrd/).
Even though cover and density of C. tenuis in the studied site has not significantly changed during these 12 years (Figure 2), changes in the dynamics of C. tenuis are evident from an increase in the number of smaller sponges (Marulanda-Gómez et al., 2017), and of new colonization cases. Despite the apparent stasis in C. tenuis population abundance, its increased coral colonization and mortality represents a long-term problem for reef recovery.
Our results showed that colonization from the substratum (S) increased for massive corals (P. strigosa, P. clivosa [Ellis and Solander, 1786], and D. labyrinthiformis). This likely occurs because these more slowly recruiting and growing species have had time to colonize dead branches of A. palmata during the 12 years lapse; the faster recruiting species like U. agaricites, M. alcicornis, and P. astreoides were the more frequent colonizers in 2002. All these colonies settling onto dead A. palmata are thus more vulnerable to be physically detached. Excavating sponges attack the exposed coral skeleton around its base, which cause the colony to dislodge, frequently dying buried by sediments (Goreau and Hartann, 1963). Carballo et al. (2008) found that around 41 % of live coral heads affected by boring sponges were completely detached. Overall, bioeroders, like clionaids, can reduce recruitment success and erode coral reef substrata, causing the breakdown of the reef framework and preventing reef recovery (Glynn and Colgan, 1992; Rützler, 2002), a process which is currently happening at Islas del Rosario.
In conclusion, C. tenuis-carrying A. palmata branches persist as vectors that disperse and translocate this sponge during heavy surge, causing further coral death. As time passes and the fragmentation and erosion of the reef increases, the evidence of the colonization of stony corals by C. tenuis through A. palmata branches vanishes, making it hard to detect previous cases by direct contact (Figure 4).











 text in
text in 




