INTRODUCTION
The worms (polychaetes and sipunculids) inhabiting the coral reefs of the Gorgona National Natural Park (Gorgona) (Figure 1) in Colombia are poorly known, mainly due to the difficulty of direct observation given the habitats they use or to the difficulty of collecting these animals. There are few studies (e.g. Cantera et al., 2003; Londoño-Cruz et al., 2003) that have mentioned some of these organisms, hence, only a few species have been identified. Some worms are considered borers (Hutchings, 1986), i.e. they penetrate (by different means) the substrate (e.g. coral) to dwell in a safe refugium. The galleries produced may weaken the substrate hardness and hence make it more fragile and prone to break; when empty these galleries may also serve as openings for the colonization of other boring species (e.g. bivalve mollusks) (Londoño-Cruz et al., 2003), which increases coral destruction.

Figure 1 Location of Gorgona Island in the TEP and the two coral reefs: Playa Blanca and La Azufrada, from which worms were collected.
Gorgona (2.9689 °N-78.1819 °W), along with Gorgonilla islet, is the largest insular territory on the Colombian Pacific coast. This island is ~30 km from the closest mainland point (Zapata, 2001; Londoño-Cruz, et al., 2003; Giraldo, 2012). Environmental conditions are marginal to coral reef formations. The mean annual precipitation is 8000 mm with concomitant low salinities that vary between 29 (during March) and 31 (during September). Water temperature varies annually and is affected by the ENSO cycle, reaching highs of 33 °C during El Niño and lows of 18 °C during La Niña. Tides are semidiurnal with a range of ~4.5 m. Finally, the concentration of total dissolved solids is relatively high, which is reflected in reduced water transparency (low visibility), this varies around 8 m year-round, with extremely low values during periods of heavy rain (Prahl and Erhardt, 1985; Zapata, 2001; Londoño-Cruz et al., 2003; Giraldo et al., 2008). Regardless of these conditions, Gorgona holds some of the most developed and diverse coral reefs in the southern reach of coral reef distribution in the TEP (Zapata, 2001; Londoño-Cruz, et al., 2003; Zapata and Vargas-Ángel, 2003; Giraldo et al., 2008).
The largest coral reefs of Gorgona, Playa Blanca and La Azufrada (9.8 and 9.4 ha, respectively), are located on the lee (southeastern side) of the island. These reefs are young and shallow (Zapata, 2001; Giraldo et al., 2008), and are dominated by Indopacific coral species (Zapata and Morales, 1997; Zapata, 2001). Playa Blanca is formed by two reef patches, the smallest of which is located to the north; in turn, La Azufrada is north to Playa Blanca (Prahl and Erhardt, 1985; Zapata, 2001) (Figure 1). These reefs show the typical zonation pattern of fringing coral reefs, with a navigation channel, followed by the back-reef, where live coral cover is less than 50%; the reef-flat, the reef-front, where live coral cover is highest, reaching more than 80 %, and the reef-slope, where live coral cover is as low as 20 % and there is abundant coral rubble (Prahl and Erhardt, 1985; Díaz et al., 2000; Zapata, 2001). Although coral richness in Gorgona is the highest in the Colombian Pacific, there is a dominance of corals of the genus Pocillopora (Prahl and Erhardt, 1985; Zapata, 2001). Given the importance of coral reef ecosystems, the scarcity in knowledge regarding worms in the Pacific coast of Colombia, and that certain species of worms may act as borers of coral substrates, the main purpose of this document was to identify the members of the boring worms associated to coral reefs of Gorgona Island (Colombian Pacific).
MATERIALS AND METHODS
Branches of Pocillopora spp. colonies, with no signs of bioerosion, were collected and shaped into similar-sized cylinders (Experimental Units: EUs), using a handheld grinder. Each EU was drilled in one of the ends to anchor a 1.59 mm diameter stainless steel L-shaped rod using epoxy resin. Besides, larger (6.35 mm diameter x 2 m long) stainless steel rods were hammered, leaving around 50 cm outside, into the reef framework. The EUs were tied (using cable ties) to this portion of the rod (one EU per rod). Five EUs were randomly deployed along each reef zone of each reef and left there during one of five different exposure periods that ranged between 6 and 18 months (with 3 month intervals). In this manner, a good spatial and temporal representation was achieved. All EUs (n=200) were deployed the same day (2016/02/11). Once the exposure period ended for an EU, it was collected, stored in a plastic container with 10% formalin, and taken to the lab. All fouling was removed from the EU surface. After that, each EU was carefully fragmented using locking pliers to extract all organisms dwelling inside the coral matrix. All worms (polychaetes and sipunculids) were identified to the lowest taxonomic level possible using dichotomic keys (Cutler, 1994; de León-González et al., 2009). Besides, original descriptions available in the Biodiversity Heritage Library were reviewed. Finally, all identified species were stored in the Reference Collection of Marine Organisms (CROM) of the Department of Biology at the Universidad del Valle, Cali (Colombia).
RESULTS
A total of 571 worm specimens (polychaetes + sipunculids) were collected (Table 1). These worms were allocated in 21 species: 18 polychaetes and 3 sipunculids (see Figure 2 for an example of some of the collected species). Polychaetes comprise 10 families and sipunculids comprise 2. New records are marked with superscripts as follows: 1for Gorgona coral reefs only, 2for PNN Gorgona, and 3for the Colombian Eastern Tropical Pacific. The consulted literature for each species is listed right below the species name. The material examined presents the batch code of the CROM. All information related to distribution was obtained from the World Register of Marine Species (WoRMS, 2019). Finally, a brief discussion is added only to species that match original descriptions but differ in certain characters.
Table 1 Total number of specimens of each species from each coral reef and reef zone (BR: Back-reef, RP: Reef-flat, RF: Reef-front, and RS: Reef-slope) collected in Gorgona Island (Colombian ETP) during the five different exposure periods.
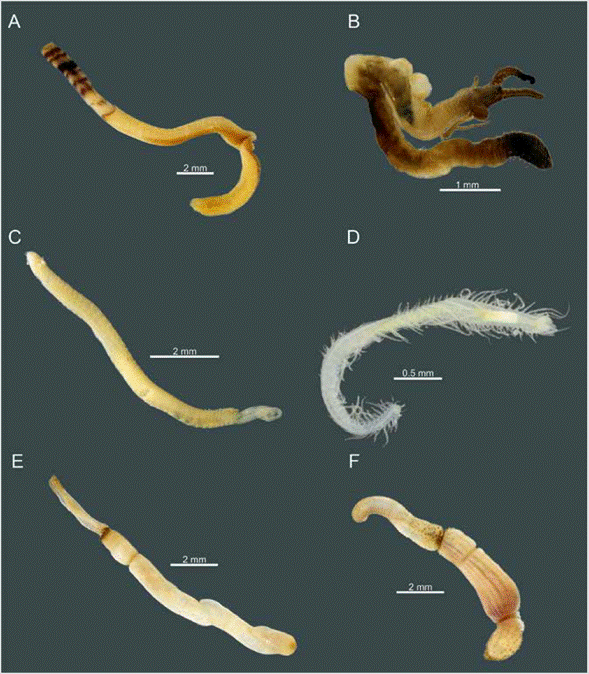
Figure 2 Six of the most common species of worms found in Gorgona Island's coral reefs. Polychaetes: A) Euratellasalmacidis. B) Dodecaceria laddi. C) Dipolydora commensalis. D) Syllis gracilis. Sipunculids: E) Aspidosiphon (Aspidosiphon) cf. elegans. F) Phascolosoma (Phascolosoma) cf. perlucens.
Annelida
Class Polychaeta
Family Amphinomidae Lamarck, 1818
Genus Chloeia Lamarck, 1818
Chloeia entypa 1Chamberlin, 1919
Chloeia entypa:Chamberlin, 1919; Kudenov, 1975; Fauchald, 1977; Prahl et al., 1979; Laverde-Castillo, 1986; Hernández-Alcántara y Solís-Weiss, 1991; Salazar-Vallejo y Londoño-Mesa, 2004; Valencia et al., 2014.
Material examined: APN0001.
Distribution: Pacific (Mexico) and TEP.
Discussion: The specimen matched the description by Kudenov (1975), who mentions the presence of serrate and bifurcate neurosetae in the posterior part of the body. Although, de León-González et al. (2009) mention that lateral antennae are pigmented. This pigmentation was not observed, probably because it was lost due to the fixation method used. It is highly probable that the specimen is a juvenile due to the number of segments present (14).
Family Cirratulidae Ryckholt, 1851
Genus Dodecaceria Örsted, 1843
Dodecaceria fewkesiBerkeley & Berkeley, 1954
Dodecaceria fewkesi:Berkeley y Berkeley, 1954; Salazar-Vallejo y Londoño-Mesa, 2004.
Material examined: CIR0026, CIR0027, CIR0028, CIR0029.
Distribution: South of California, Coos Bay.
Genus Dodecaceria Örsted, 1843
Dodecaceria laddi 1Hartman, 1954
Dodecaceria laddi:Hartman, 1954; Gibson, 1978; White, 1980; Abd-Elnaby, 2009; Magalhaes y Bailey-Brock, 2013.
Material examined: CIR0030, CIR0031, CIR0032,
CIR0033, CIR0034, CIR0035.
Distribution: Kaneohe Bay (Hawaii), Marshall Islands, Pulo Boenda (Sumatra).
Discussion: The specimens matched the description by Hartman (1954); however, it was not possible to observe the limbo that appears in the setae of the first seven segments (Hartman, 1954, Figure 177H). This might be due to the position of the setae.
Genus Timarete Kinberg, 1866
Timarete luxuriosa 3 (Moore, 1904)
Timarete luxuriosa:Moore, 1904; Hernández-Alcántara y Solís-Weiss, 1991; Hernández-Alcántara, 2003; Salazar-Vallejo y Londoño-Mesa, 2004; Choi et al., 2018.
Material examined: CIR0036, CIR0037.
Distribution: Gulf of California, Mexican Pacific, TEP.
Family Dorvilleidae Chamberlin, 1919
Genus Dorvillea Parfitt, 1866
Dorvillea rubra 3, (Grube, 1856)
Dorvillea rubra: Grube, 1856; Perkins, 1998; Salazar-Vallejo y Londoño-Mesa, 2004; Hernández-Alcántara et al., 2014.
Material examined: DOR0001, DOR0002, DOR0003, DOR0003, DOR0004, DOR0005, DOR0006.
Distribution: Caribbean, Florida, Gulf of Mexico, TEP.
Family Eunicidae Berthold, 1827
Genus Eunice Cuvier, 1817
Eunice cedroensis 3Fauchald, 1970
Eunice cedroensis:Fauchald, 1970; Salazar-Vallejo y Londoño-Mesa, 2004.
Material examined: EUN0035, EUN0036, EUN0037, EUN0038.
Distribution: Gulf of Mexico, TEP.
Discussion: Although the specimens matched the description by Fauchald (1970), the setae illustrated in Figure E (Plate 2) were not observed.
Genus Lysidice Lamarck, 1818
Lysidice ninetta 1 Audouin & H Milne Edwards, 1833
Lysidice ninetta:Audouin y Milne, 1833; Monro, 1928; Fauchald, 1970, 1977; Laverde-Castillo, 1986; Hernández-Alcántara y Solís-Weiss, 1991; de León-González et al., 1993; Gómez et al., 1997; Cantera et al., 1999; Salazar-Vallejo y Londoño-Mesa, 2004.
Material examined: EUN0040, EUN0041, EUN0042
Distribution: TEP, Colombia, Gulf of Mexico, Cuba, Belize, Belgium, Caribbean Sea, France, Greece, Jamaica, New Zealand, Red Sea, Spain, United Kingdom.
Genus Palola Gray in Stair, 1847
Palola paloloides 2 (Moore, 1909)
Palola paloloides:Moore, 1909; Fauchald, 1970, 1992; Kudenov, 1975; Laverde-Castillo, 1986; Hernández-Alcántara y Solís-Weiss, 1991; Salazar-Vallejo y Londoño-Mesa, 2004.
Material examined: EUN0043.
Distribution: San Diego, California; TEP, Colombian Pacific.
Family Flabelligeridae de Saint-Joseph, 1894
Genus SemioderaChamberlin, 1919
Semiodera cariboum 3 (Grube, 1859)
Semiodera cariboum:Hartman, 1956; Salazar-Vallejo y Londoño-Mesa, 2004.
Material examined: FLA0006.
Distribution: Antilles, Caribbean Sea, Gulf of Mexico, TEP.
Discussion: Salazar-Vallejo and Londoño-Mesa (2004) register this as a dubious species for the TEP (S. cariboa); however, the observed specimen matched the descriptions by Grube (1858) and de León-González et al. (2009).
Family Nereididae Blainville, 1818
Genus Eunereis Malmgre, 1865
Eunereis cf. longipes 3Hartman, 1936
Eunereis cf. longipes:Hartman, 1936; Salazar-Vallejo y Londoño-Mesa, 2004.
Material examined: NER0001, NER0002, NER0003, NER0004.
Distribution: California, TEP.
Discussion: Observed specimens matched the description by Hartman (1936); however, although having notopodial laminar homogonfous falcigerous ending in an acute tip (Hartman, 1936, Figure 53), the falcigerous lamina is longer and the tooth more acute. Likewise, in Figure 53F (Hartman, 1936), teeth can be seen along the whole setae sheet, while in the Colombian specimens, the teeth were only observed in the distal portion of the setae and the first tooth is longer and surrounds the falcigerous tooth.
Family Phyllodocidae Örsted, 1843
Genus Eteone Savigny, 1822
Eteone cf. pacifica 3Hartman, 1936
Eteone cf. pacifica:Reish, 1963; Salazar-Vallejo y Londoño-Mesa, 2004.
Material examined: PHY0008, PHY0009.
Distribution: Friday Harbor (San Juan Island), TEP.
Discussion: Observed specimens matched the description by de León-González et al. (2009); however, ventral and dorsal cirri differ in longitude and lack a well-defined auricular shape. This can be due to slight changes in soft tissues during fixation.
Family Sabellidae Latreille, 1825
Genus EuratellaChamberlin, 1919
Euratella salmacidiss (Claparède, 1869)
Euratella salmacidis:Claparède, 1869; de León-González et al., 1993; Salazar-Vallejo y Londoño-Mesa, 2004.
Material examined: SAL0005, SAL0006, SAL0007, SAL0008, SAL0009, SAL0010.
Distribution: Mediterranean Sea, North Atlantic Ocean, Spain, TEP.
Family Spionidae Grube, 1850
Genus Dipolydora Verril, 1881
Dipolydora commensaliss (Andrews, 1891)
Dipolydora commensalis:Andrews, 1891; Hartman, 1941; Salazar-Vallejo y Londoño-Mesa, 2004.
Material examined: SPI0011, SPI0012, SPI0013, SPI0014, SPI0015, SPI0016.
Distribution: North Carolina, Japan Sea, North Atlantic Ocean, TEP.
Genus Polydora Bosc, 1802
Polydora ciliata 3 (Johnston, 1838)
Polydora ciliata: Johnston, 1838; Hartman, 1941; Mustaquim, 1986; Salazar-Vallejo y Londoño-Mesa, 2004.
Material examined: SPI0019.
Distribution: United Kingdom, French, Gulf of Naples, Black Sea, Gulf of Aden, North Atlantic Ocean, Belgium, Bay of Fundy, English Channel, Greece, Ireland, Madagascar, Mozambique, Netherlands, North Sea, Red Sea, Spain, Ukraine, TEP.
Discussion: The observed specimen matched the descriptions by Johnston (1838) and Mustaquim (1986), with the only exception that the four eyes reported by Mustaquim (1986) were not observed in our specimen.
Family Syllidae Grube, 1850
Genus CiceseDíaz-Castañeda & San Martín, 2001
Cicese cf. sphaerosylliformis 3 Díaz-Castañeda & San Martín, 2001
Cicese cf. sphaerosylliformis:Díaz-Castañeda y San Martín, 2001; Díaz-Castañeda et al., 2005; Hernández-Alcántara et al., 2008.
Material examined: SYL0011.
Distribution: Pacific Coast of Baja California (Mexico).
Discussion: The observed specimen matched the description in Díaz-Castañeda and San Martín (2001), but the distance between distal and basal falcigerous teeth is shorter than the one presented in their illustration. Besides, in the simple setae, there are no teeth present as shown in the illustration by Díaz-Castañeda and San Martín (2001, Figure 5G, 5H, 5I).
Genus Dentatisyllis Perkins, 1981
Dentatisyllis cf. carolinae 1 (Day, 1973)
Dentatisyllis cf. carolinae:Day, 1973; Perkins, 1980; de León-González et al., 2009.
Material examined: SYL0012, SYL0013.
Distribution: Gulf of Mexico, North Atlantic Ocean.
Discussion: de León-González et al. (2009) register a trepan with approximately 10 teeth, while in our specimens, only 8 teeth were observed. For the articulated dorsal cirri, they mention 30 segments, while in our specimens we observed only approximately 20 segments. Finally, the simple setae illustrated by Day (1973, Figure 4E) and Perkins (1980, Figure 38A) were not observed in our specimens.
Genus Syllis Lamarck, 1818
Syllis gracilis 1Grube, 1840
Syllis gracilis:Grube, 1840; Monro, 1933; Fauchald, 1977; Laverde-Castillo, 1986; Salazar-Vallejo et al., 1990; Estape y San Martín, 1991; Hernández-Alcántara y Solís-Weiss, 1991; de León-González et al., 1993; Bastida-Zavala, 1995; Capa et al., 2001; Hernández-Alcántara et al., 2003; Salazar-Vallejo y Londoño-Mesa, 2004.
Material examined: SYL0018, SYL0019, SYL0020, SYL0021, SYL0022.
Distribution: Gulf of Naples, Mediterranean Sea, Black Sea, Chilean part of the South Pacific Ocean, Djiboutian region of the Gulf of Aden, Djiboutian region of the Red Sea, Antilles, West Africa Coast, Gulf of Mexico, Caribbean Sea, Trinidad and Tobago, Spain, United Kingdom, Italy, Ireland, Greece, TEP.
Genus Syllis Lamarck, 1818
Syllis prolifera 3 Krohn, 1852
Syllis prolifera:López et al., 1997; Capa et al., 2001; Salazar-Vallejo y Londoño-Mesa, 2004.
Material examined: SYL0017.
Distribution: Antilles, Black Sea, Djiboutian part of the Gulf of Aden, Djiboutian part of the Red Sea, Caribbean Sea, Cuba, Gulf of Mexico, Italy, Madagascar, New Zealand, North Atlantic Ocean, Spain, Trinidad and Tobago, United Kingdom, Greece, TEP.
Sipuncula
Class Phascolosomatidea
Family Aspidosiphonidae Quatrefages, 1865
Genus Aspidosiphon Diesing, 1851
Aspidosiphon (Aspidosiphon) cf. elegans 3 (Chamisso & Eysenhardt, 1821)
Aspidosiphon (Aspidosiphon) cf. elegans:Chamisso y Eysenhardt, 1821; Cutler, 1994.
Material examined: ASP0001, ASP0002, ASP0003,
ASP0004, ASP0005, ASP0006, ASP0007.
Distribution: Mediterranean Sea-Eastern Basin, Aegean Sea, Gulf of Mexico, Madagascar, North Atlantic Ocean, Red Sea, South Pacific Ocean.
Family Phascolosomatidae Stephen & Edmonds, 1972
Genus Phascolosoma Leuckart, 1828
Phascolosoma (Phascolosoma) cf. nigrescens 3 (Keferstein, 1865)
Phascolosoma (Phascolosoma) cf. nigrescens:Fisher, 1952; Cutler, 1994.
Material examined: PHS0001, PHS0002, PHS0003. Distribution: Madagascar, Red Sea, Gulf of Mexico.
Phascolosoma (Phascolosoma) cf. perlucens Baird, 1868
Phascolosoma (Phascolosoma) cf. perlucens:Cutler, 1994; Cantera et al., 2003; Kawauchi y Giribet, 2010.
Material examined: PSH0004, PSH0005, PSH0006, PSH0007, PSH0008, PSH0009, PSH0010.
Distribution: Madagascar, Gulf of Mexico, Gorgona Island (Colombia).
Discussion: The observed specimens matched the description by Kawauchi and Giribet (2010); however, we use cf. because, according to the aforementioned authors, there is a need for molecular analyses to discern this specie within the genus since morphological characters are highly variable and not fully useful for the correct identification of species.











 text in
text in 


