INTRODUCTION
The Spongiidae family includes sponges that are used commercially for bathing and washing. These are characterized by having a skeleton of homogeneous spongin fibers, without distinctive laminations, generally dominated by a network of sub-primary fibers, and also by having diplodal choanocytes chambers. This family consists of six valid genera, one of which, SpongiaLinnaeus, 1759, includes three subgenera: Spongia, HeterofibriaCook, and Bergquist, 2001 and AustralospongiaCook and Bergquist, 2001 (Cook and Bergquist, 2001). Species of the genus Spongia possess a surface of uniformly arranged low conules. The consistency is elastic, flexible, and very compressible. The skeletal reticulum is composed of a small number of ascending primary fibers cored with foreign material and a highly developed uncored secondary reticulum (Cook and Bergquist, 2002). Spongia contains 65 valid described species, of which 8 are found in the Caribbean (Van Soest et al., 2019).
The subgenus Heterofibria is distinguished from the other two subgenera by having a distinctive size dichotomy between the fibers of the secondary skeleton. Fibers with a larger diameter are called secondary and those with a smaller diameter pseudo-tertiary, which generates a sub-primary reticulum (Cook and Bergquist, 2001). The first species of the subgenus Heterofibria described were S. corrugataCook and Bergquist, 2001, S. decookiVan Soest and Hooper, 2020 (same as Spongia (Heterofibria) cristataCook and Bergquist, 2001), S. gorgonocephalus Cook and Bergquist, 2001, S. manipulatusCook and Bergquist, 2001 and S. mokohinauCook and Bergquist, 2001 in New Zealand, based on a review of the genus Spongia (Cook and Bergquist, 2001). This subgenus is represented in the Brazilian Atlantic by S. catarinensisMothes, Kasper, Lerner, Campos, and Carraro, 2006. Kim and Sim (2009) described two species from the coasts of Korea: S. corallina and S. purpurea.Samaai et al. (2019) described S. cooki (a name that was replaced by S. peddemorsiSamaai, Pillay and Janson, 2020) and S. smaragdus from Sodwana Bay, on the east coast of South Africa.
For Colombia, two species of Spongia have been recorded and described by Zea (1987): S. (Spongia) tubuliferaLamarck, 1814 and S. (Spongia) ancloteaDe Laubenfels and Storr, 1958 (as S. obscuraHyatt, 1877 and S. pertusaHyatt, 1877, respectively). This study describes a new species of Spongia (Heterofibria) collected in breakwaters from the beaches of the department of Sucre (Colombian Caribbean).
STUDY AREA
This study was carried out in two calcareous rock breakwaters located on the Boca La Caimanera beaches (9°26'6.33"-21.46" N, 75°37'40.39"-52.12") and Punta Piedra (9°25'21.62" N, 75°39'5.31" W) in the municipality of Coveñas, department of Sucre (Colombia), the southern part of the Gulf of Morrosquillo (Figure 1). The breakwaters are located on both sides of the mouth of the coastal lagoon La Caimanera, at a maximum distance of 116 m from the coast and at a depth of less than 3 m. Breakwaters are accompanied by seagrass meadows inhabited by mollusks, echinoderms, and porifers. Punta Piedra is a coral reef composed mainly of the species Millepora complanata Lamarck, M. squarrosa Lamarck, Siderastrea siderea (Ellis and Solander), and Siderastrea radians (Pallas); it is deteriorated and dead coral is abundant. The wave height is lower than that found in other parts of the Colombian Caribbean and extreme wave events are generated mainly by cold fronts (Otero et al., 2016).
materials and methods
The specimens were collected between August 20, 2017 and June 22, 2018 by free diving, manually using a spatula, and placed in a resealable bag containing seawater. Photographs were also taken on-site with an SJCAM SJ4000 camera at 12 mp. The samples were fixed and labeled in glass jars with 70 % ethanol for subsequent taxonomic identification and, later, they were deposited in the Museum of Zoology of the University of Sucre (MZUSU).
The identification of the sponges was carried out through thick histological sections according to the methodology of Zea (1987). The taxonomic keys proposed by Hooper and Van Soest (2002b) and the work of Mothes et al. (2006) up to the subgenus level. Comparisons were also made with samples of the species S. anclotea, S. tubulifera and Hyatella cavernosa (Pallas, 1766) to arrive at the species level. The photographs of the skeleton architecture were taken with a Labomed microscope equipped with an iVu 3100 camera. The measurements of the fibers and the distance between them were calculated with the PixelPro version 2.8 program, from the calibration of the software with a micrometer of the platen. Measurements are presented as minimum-average-maximum. On the other hand, the measurements of the oscula, the lobes, and the size of the fragment were calculated with an electronic caliper.
RESULTS
Taxonomy
Class Demospongiae Sollas, 1885
Order Dictyoceratida Minchin, 1900
Family Spongiidae Gray, 1867
Genera SpongiaLinnaeus, 1759
Diagnosis (from Cook and Bergquist, 2001): soft to firm and compressible sponges. With a skeletal network of primary fibers, cored with foreign material and uncored secondary fibers; more obvious primary fibers near the surface, where they can pass through the dermis. Conules, when present, are supported by one or more tufts of emerging primary fibers. Skeleton is dominated by a mesh of uncored secondary fibers, which gives these sponges their flexibility and their water retention properties. These meshes are created by the intersection and union of fibers, and each fiber intersection always has three fibers moving away from it.
Subgenus HeterofibriaCook and Bergquist, 2001
Diagnosis (from Cook and Bergquist, 2001): skeleton dominated by networks of sub-primary fibers, of which the larger diameter fibers are called secondary fibers and the smaller diameter fibers pseudo-tertiary. Meshes formed by skeleton fibers, usually polygonal, although irregular in size and shape, less angular than those of the subgenus Spongia, as characterized in S. officinalis.
Spongia (Heterofibria) sucrensis sp. nov. (Figure 2)
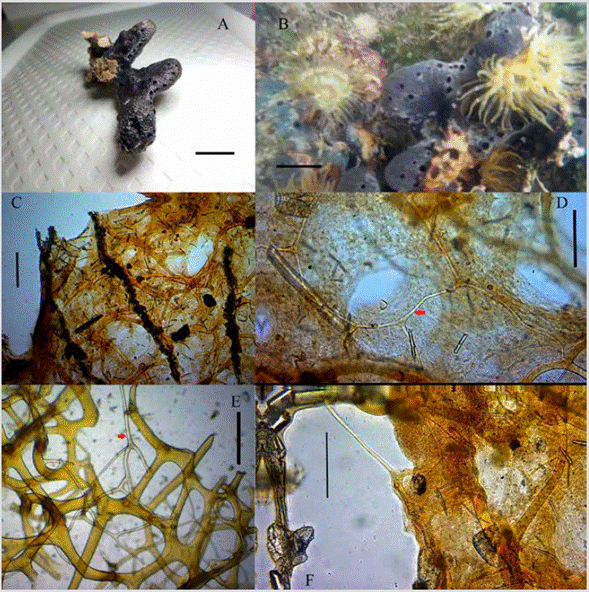
Figure 2 Spongia (Heterofibria) sucrensis sp. nov. A) Preserved specimen (scale: 3 cm). B) Specimen in situ (scale: 3 cm). C) Architecture of the skeleton, primary fibers cored with of foreign material that emerge towards the surface forming a conule (scale: 300 µm). D) Architecture of the skeleton with organic material, the reticulum of pseudo-tertiary fibers (red arrow) (scale: 200 µm). E) Architecture of the skeleton without organic material, the reticulum of pseudo-tertiary fibers (red arrow) (scale: 200 µm). F) Solitary pseudo-tertiary fiber (scale: 200 µm).
Material type: Holotype. MZUSU-I00005: Boca La Caimanera, breakwater, 1-3 m, August 20, 2017, col. J. David; Paratypes: MZUSU-I00026: Punta Piedra, coral reef, 2-4 m, June 22, 2018, col. J. David; MZUSU-I00027: Boca La Caimanera, breakwater, 1-3 m, August 20, 2017, col. J. David; MZUSU-I00028: Punta Piedra, coral reef, 2-4 m, June 22, 2018, col. J. David.
Additional material for comparison: Spongia (Spongia) tubulifera (MZUSU-I00014); Hyattella cavernosa (MZUSU-I00040); S. (Spongia) anclotea (MZUSU-I00036).
Diagnosis: massive sponge, with erect and creeping lobes or branches. Skeleton of ascending primary fibers cored with foreign material. Meshes of uncored secondary and pseudo-tertiary fibers dominate the skeleton. Lighter pseudo-tertiary fibers are connected to the other types of fibers or forming a reticulum within the meshes of secondary fibers.
External morphology: massive sponge with erect, creeping lobes of a maximum length of 5 cm, and a maximum width of 1.7 cm. Round oscula as perforations of the pinacoderm, 1-3 mm in diameter, located mainly on the upper part of the lobes and arranged linearly along with the sponge. Surface covered by conules of height between 0.3 and 0.8 mm. Some of the conules have a fine fiber protruding from the apices. Elastic and compressible consistency, difficult to tear. Color in situ black in the upper part and creamy to white in the basal and interior area of the sponge; the color is kept in alcohol. Maximum dimensions: 11.5 x 4.7 x 1.9 cm (length-height-width) (Figure 2A).
Ectosome: distinguishable organic pinacoderm.
Coanosoma: anisotropic skeleton, not very dense, sometimes with much debris, of ascending primary fibers cored with foreign material (spicules and debris); many of these fibers bifurcate into thinner sections as they approach the surface, where the distance between them is less (Figure 2C). Uncored secondary and pseudo-tertiary fibers dominate the reticulated skeleton and are connected to other fibers forming meshes. The secondaries are frequently formed irregular polygons in terms of size and shape while the pseudo-tertiary ones are found alone, connected with the other types of fibers or forming a reticulum only of these, but that connects with the other types of fibers; this frequently occurs near the surface (Figure 2D, 2F). This reticulum is found within meshes of the other fibers, parallel to these, although some fibers can twist in another direction and connect with another group of meshes (Figure 2D, 2E). Yellow coloration of the primary and secondary fibers, in pseudo-tertiary is clearer. Fiber diameter: primary 48-80.2-119 µm (n = 33); secondaries 19-41.3-81 µm (n = 30); pseudo-tertiary 7-13.9-24 µm (n = 30). Distance between primary fibers: 204-688-1401 µm (n = 28). Diameter of the polygons formed by primary, secondary and pseudo-tertiary fibers: 72-300-801 µm (n = 30); reticulum polygons of pseudo-tertiary fibers: 60262-563 µm (n = 20).
Ecology: Found in the artificial rocky substrate (breakwater) and on dead coral at a depth of approximately 1.5 m. Inside a sponge, two bivalves of the genus Mytilopsis were found; Anemones of the genus Exaiptasia were found next to the sponge.
Distribution: Sucre coast (Colombian Caribbean).
Etymology: The specific name refers to the department of Sucre, where it was collected.
DISCUSSION
According to Van Soest et al. (2019), the genus Spongia has so far eight species registered for the Caribbean: S. (Spongia) obliquaDuchassaing and Michelotti, 1864, S. (Spongia) barbaraDuchassaing and Michelotti, 1864, S. anclotea, S. (Spongia) coelosiaDuchassaing and Michelotti, 1864, S. (Spongia) obscuraHyatt, 1877, S. (Spongia) pilosa (Wilson, 1902), S. (Spongia) solitariaHyatt, 1877 and S. tubulifera. Of these, S. tubulifera and S. anclotea (as S. obscura and S. pertusa, respectively) have been recorded and described in different areas of the Colombian Caribbean (Zea, 1987). Therefore, with the description of S. sucrensis the record is extended to nine species of the genus for the Caribbean and three for Colombia. Similarly, it is the first time that the subgenus Heterofibria has been recorded in the Caribbean. Previously, the subgenus had only been recorded in South America on the Ilha das Aranhas in the state of Santa Catarina (Brazil) (Mothes et al., 2006; Muricy et al., 2011), in New Zealand (Cook and Bergquist, 2001), in Korea (Kim and Sim, 2009) and on the east coast of South Africa (Samaai et al., 2019).
This new species is close to Spongia (Heterofibria) catarinensis (Mothes et al., 2006), but its external morphology differs from this in that the laterally scattered projections are elevated; it does not feature oscula located in discrete raised lobed projections of its surface; the microconules are smaller (0.3-0.8 mm); the maximum diameter of the oscula is greater (3 mm by 2 mm in S. catarinensis) and there is no translucent membrane surrounding the opening of the oscula. Regarding the skeleton, near the surface, the new species has reticles formed by pseudo-tertiary fibers that connect with the adjacent primary and secondary fibers. These reticles are absent in S. catarinensis. The measurements carried out show that the maximum diameter of the primary and secondary fibers is greater in the new species, while the pseudo-tertiary fibers show great similarity (Table 1). The reticulum of pseudo-tertiary fibers has also been mentioned in other species of the subgenus that have a distant distribution with respect to the new species. Due to the short duration of sponge larvae, it is highly unlikely that they are (Klautau et al., 1999). Spongia (H.) peddemorsi [equal to S. cooki in Samaai et al. (2019)], a species from South Africa, also possesses a reticulum of pseudo-tertiary fibers connected with secondary fibers, but it differs from the new species in the presence of a superficial collagen layer and smaller fiber diameters (primary, 55-65-89 um, secondary 23-28-37 um) (Table 1). In the same way, the new species differs from S. (H.) corallina and S. (H.) purpurea in that their peudo-tertiary fibers are connected to a dermal membrane and not to primary or secondary fibers (Kim and Sim, 2009). For the New Zealand species presented in Cook and Bergquist (2001), the new species is similar in skeletal architecture to S. (H.) decooki [same as S. (H.) cristata] and S. (H.) mokohinau, but in external morphology, it differs consistently. The external morphology is a character that can vary in some species of sponges depending on the habitat. However, in this case, morphology has been used to differentiate the species of this subgenus (Cook and Bergquist, 2001; Samai et al., 2019). In the present work, the shape of the oscula of the new species is used as a differentiating character, since, both in the holotype and in the paratypes, despite being from different habitats, the oscula as perforations of the dermis, so it seems to be a consistent character.
Table 1 Diagnostic characteristics of the subgenus Heterofibria species. Diameters of PR: Primary fibers, SC: Secondary fibers, PT: Pseudo-tertiary fibers.

This species differs from S. tubulifera and S. anclotea, which are also found in the Colombian Caribbean, mainly due to the presence of pseudo-tertiary fibers. Likewise, its fibers are larger in diameter and more irregular in architecture than in S. tubulifera and its external morphology does not present the oscular tubules characteristic of this. Spongia anclotea, in addition to having smaller diameter fibers, is highly condensed, so that only the primary fibers can be distinguished by cored of foreign material. Finally, although H. cavernosa has small diameter fibers that could be called pseudo-tertiary, these are larger in diameter than those of the new species. The primary fibers are at a greater distance from each other and are often uncored. Also, H. cavernosa has deep subdermal spaces that are absent in the new species.











 text in
text in 



