INTRODUCTION
In the Caribbean Sea and the Venezuelan Southeastern Caribbean, the copepod community is the most numerous and diverse mesozooplankton group which reports the largest number of spatial-temporal distribution studies. However, some estimations are constrained in determining their overall distribution and abundance (Calef and Grice, 1967; Zoppi 1977; Márquez-Rojas and Marín, 2007), and there are few works which are known to include taxonomy (Cervigón, 1963; Legaré, 1964; Cervigón and Marcano, 1965; Owre and Foyo, 1967; Michel and Foyo, 1976). These studies are limited to the country’s northeastern coastal area, mainly the Cariaco Gulf and Basin (Legare, 1961, 1964; Zoppi, 1961; Cervigón, 1963; Cervigón and Marcano, 1965; Márquez-Rojas et al., 2006, 2014a, 2014b, 2020). Therefore, there are no current estimations of this group’s diversity in the northeastern region of Venezuela.
The limitations associated with an inaccurate knowledge of the identified copepods may be partly due to continuous changes in the group’s systematics. In addition, the small morphological differences between some species contribute to species identification errors in many studies. The great diversity of shapes that a species takes before reaching the adult stage (six nauplii, four or five copepodite stages, and the adult copepod) is among the reasons why it is difficult to characterize certain species (Miracle, 2015). All the above may have had an influence in the lack of knowledge about this group in Venezuela, together with the limited number of Venezuelan scientists dedicated to the taxonomic study of this crustacean subclass (Liñero-Arana et al., 2009).
Based on the above-presented evidence, and with the aim of unifying criteria regarding copepod diversity in the northeastern region of Venezuela, we present an updated and revised list of the copepods recorded in the five major areas in which this region has been divided: Mochima Bay (BM), Cariaco Basin (CC), Gulf of Cariaco (GC), Northeastern Caribbean Sea (MCN), and the Paria Peninsula and Gulf (PGP) (Fig. 1). Therefore, this research allows identifying information gaps and orienting future lines of research in the field of copepods.
MATERIALS AND METHODS
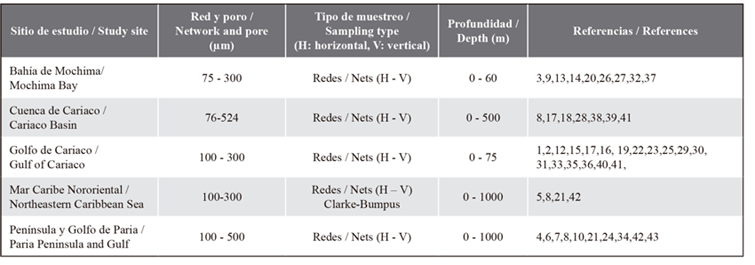
The bibliographic sources included in this list are all national and international publications that provide an inventory of copepods identified at least at the species level, although a few of them that include genera are presented. Diverse studies (taxonomic and ecological as well as lists) published between 1961 and 2020 were considered. Data from sources with limited accessibility are also included, i.e., undergraduate, Master’s, and doctoral works and final research project reports. The review was based on approximately 50 works, out of which five were intended to clarify synonymies, to include relevant notes, or to generally obtain data. From the reviewed documents, we extracted information regarding the types of zooplankton nets and the mesh sizes used, the sampling type, and the collection depth, in addition to the published and reviewed studies on each of the areas under analysis (Table 1).
Table 1 Methodological aspects of the sampling performed in zooplanktonic studies that extracted copepods in the northeastern region and the Atlantic of Venezuela. 1: Bagdo (1977), 2: Bastardo (1975) , 3: Brito (2013), 4: Calef y Grice (1967), 5: Caraballo (1976), 6: Cervigón (1963), 7: Cervigón (1964), 8: Cervigón and Marcano (1965), 9: Colina (2019), 10: Cova (2018), 12: Espinoza (1977), 13: Expósito (1997), 14: González (2003), 15: Infante and Urosa (1986), 16: Kim et al. (2019), 17: Legaré (1961), 18: Legaré (1964), 19: Marcano (2007), 20: Marcano et al. (2010), 21: Márquez-Rojas (2005), 22: Márquez-Rojas (2010), 23: Márquez-Rojas (2016), 24: Márquez-Rojas and Marín (2007), 25: Márquez-Rojas et al. (2006), 26: Márquez-Rojas et al. (2007), 27: Márquez-Rojas et al. (2008), 28: Márquez-Rojas et al. (2009), 29: Márquez-Rojas et al. (2011), 30: Márquez-Rojas et al. (2014a), 31: Márquez-Rojas et al. (2014b), 32: Márquez-Rojas and Zoppi (2017), 33: Márquez-Rojas et al. (2020), 34: Martín et al. (2007), 35: Morales (2008), 36: Morales (2014), 37: Narváez et al. (2019), 38: Peñuela (2000), 39: Pineda-Polo (1979), 40: Serrano (2015), 41: Zoppi (1961), 42: Zoppi (1977), 43: Zoppi et al. (2008).
Once the database was complete, the scientific names were updated according to the nomenclature in the WoRMS Editorial Board website (2023), which is based on Boxshall and Halsey (2004) and Walter and Boxshall (2021). The spatial distribution of each species is presented while considering the five major areas in which the northeastern region of the country has been divided, as well as the works reporting them (Table 2).
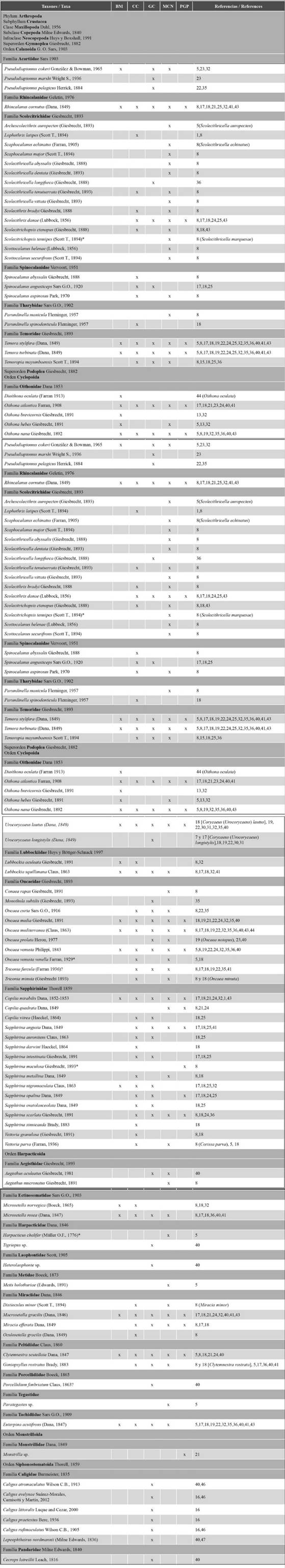
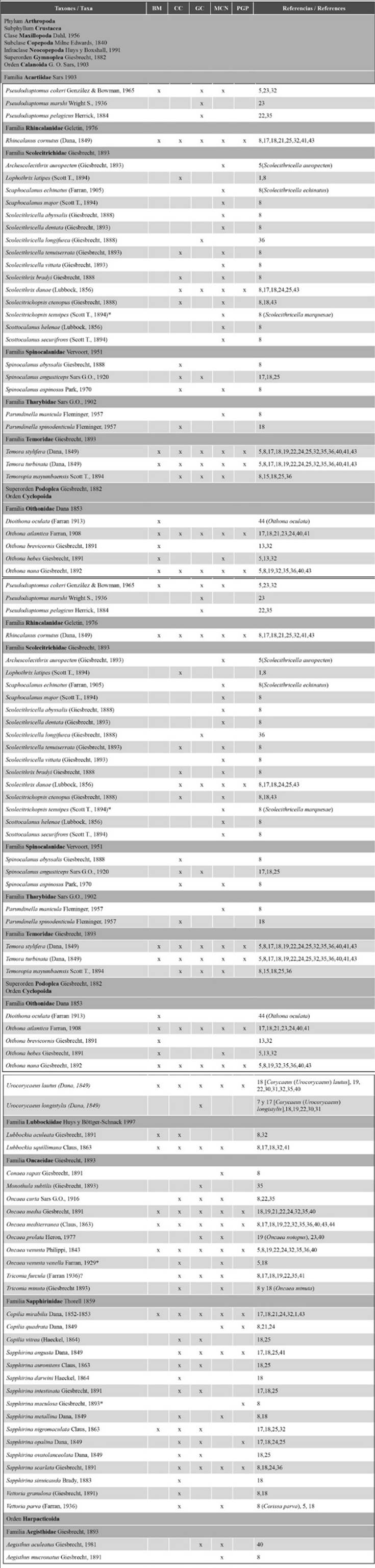
Table 2 Taxonomic list of the copepod species identified in the five areas of the northeast coast of Venezuela: Mochima Bay (BM), Cariaco Basin (CC), Gulf of Cariaco (GC), Northeast Caribbean Sea (MCN) and the Peninsula and Gulf of Paria (PGP). *Species cited in their respective works for the first time for Venezuela and the Caribbean Sea; (?) unconfirmed record. 1: Bagdo (1977), 2: Bastardo (1975), 3: Brito (2013), 4: Calef y Grice (1967), 5: Caraballo (1976), 6: Cervigón (1963), 7: Cervigón (1964), 8: Cervigón y Marcano (1965), 9: Colina (2019), 10: Cova (2018), 12: Espinoza (1977), 13: Expósito (1997), 14: González (2003), 15: Infante y Urosa (1986), 16: Kim et al. (2019), 17: Legaré (1961), 18: Legaré (1964), 19: Marcano (2007), 20: Marcano et al. (2010), 21: Márquez-Rojas (2005), 22: Márquez-Rojas (2010), 23: Márquez-Rojas (2016), 24: Márquez-Rojas y Marín (2007), 25: Márquez-Rojas et al. (2006), 26: Márquez-Rojas et al. (2007), 27: Márquez-Rojas et al. (2008), 28: Márquez-Rojas et al. (2009), 29: Márquez-Rojas et al. (2011), 30: Márquez-Rojas et al. (2014a), 31: Márquez-Rojas et al. (2014b), 32: Márquez-Rojas y Zoppi (2017), 33: Márquez-Rojas et al. (2020), 34: Martín et al. (2007), 35: Morales (2008), 36: Morales (2014), 37: Narváez et al. (2019), 38: Peñuela (2000), 39: Pineda-Polo (1979), 40: Serrano (2015), 41: Zoppi (1961), 42: Zoppi (1971), 43: Zoppi (1977), 44: Zoppi (1999), 45: Zoppi et al. (2008), 46: Suárez-Morales et al. (2012), 47: Díaz-Díaz (2000) .
Some specimens of the different copepod species have been catalogued and deposited in the reference collection of the Ecology and Taxonomy Lab of the Oceanographic Institute of Venezuela (LEZ-IOV).
Description of the study areas
Mochima Bay (BM): It is of great importance and ecological interest. It is one of the most fertile marine ecosystems in the country, as it is located in a coastal upwelling area (Okuda et al., 1968; Quintero et al., 2004; Fig. 1).
Cariaco Basin (CC): It is the world’s largest anoxic basin and has very high nutrient concentrations. With an elongated shape, it is made up of two great depressions. The western depression is the largest (78 km long and 35 km wide) and the deepest one (1435 m). Both depressions are joined by a Central Sill (915 m). And the eastern one is much smaller (76 km long and 18 km wide), with a maximum depth of 1350 m.
Gulf of Cariaco (GC): It has an elongated elliptical shape, an average surface of 642 km2, and average and maximum depths of 50 and 90 m, respectively. It is approximately 62 km long and 15 km wide (Okuda et al., 1978). It is connected to the Caribbean Sea by means of its western mouth through a narrow 5 km pass.
Northeastern Caribbean Sea (MCN): It encompasses Venezuela’s largest continental shelf, and it is 80-100 km wide and 10-30 m deep. These depths increase towards the East, where they reach values between 100 and 500 m (Fig. 1).
Paria Peninsula and Gulf (PGP): The Paria Peninsula is a well-defined upwelling focus that is influenced by the open sea, and the Gulf of Paria shows a continuous water recirculation. Both of them are influenced by the Amazon-Orinoco plume, which is lower in the former and higher in the latter. Generally speaking, this entire region is characterized by a high primary productivity (Gómez, 2001; Martín et al., 2007; Fig. 1).
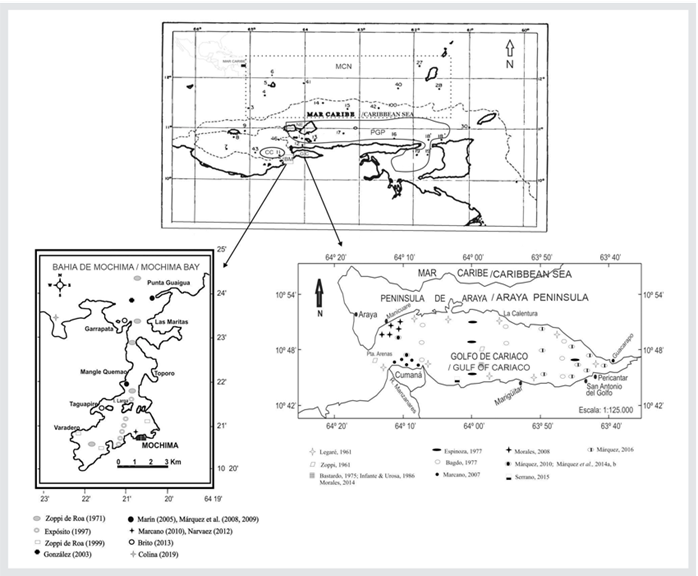
Figure 1 Geographical location of the five study areas in the northeastern zone of Venezuela. For Mochima Bay (BM) and the Gulf of Cariaco (GC), the seasons analyzed by different authors are shown. The Northeastern Caribbean Sea (MCN) is indicated with a dotted line, and the Paria Peninsula and Gulf (PGP) is shown with a solid line. The numbers indicate the seasons comprised by Cervigón and Marcano’s study (19+65).
RESULTS AND DISCUSSION
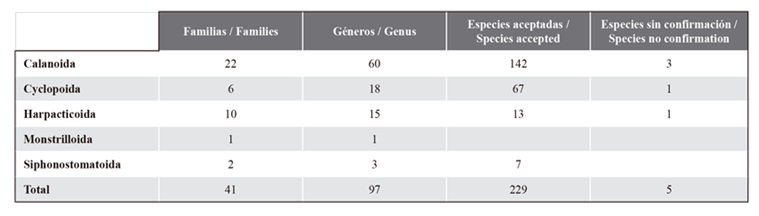
Table 2 presents the taxonomic list of species of the subclass Copepoda recorded in neritic, oceanic, and estuarine waters of the Venezuelan North-East and Atlantic. From this subclass, the orders Calanoida, Cyclopoida, Harpacticoida, Siphonostomatoida, and Monstrilloida have been recorded for the study area. These orders include 41 families, 97 genera, and 234 species. The families and species were distributed as described for each order: Calanoida: 22 families and 142 species; Cyclopoida: 6 and 67; Harpacticoida: 10 and 13; Siphonostomatoida: 2 and 7; and one genera of Monstrilloida (Table 3).
Table 3 Number of families, genera and species of the orders of copepods of the northeastern Caribbean and Venezuelan Atlantic.
Mochima Bay (BM)
In Mochima Bay, 75 species have been identified to date, which are distributed in four orders and 26 families (Table 2). These represent approximately 33% of the taxa counted for the copepod fauna in the country’s North-East (Márquez-Rojas and Zoppi, 2017).
The largest number of species (44) identified in this bay corresponds to the order Calanoida. Among the most frequent genera, Acartia, Temora, Calanus, Paracalanus, Labidocera, and Corycaeus are mentioned (Márquez-Rojas et al., 2007, 2008; Márquez-Rojas and Zoppi, 2017). Expósito (1997) reported Paracalanus quasimodo, Oithona nana, and P. cokeri as the most abundant species, whereas González (2003) found Clausocalanus arcuicornis, Acartia danae, Corycaeus speciosus, Euterpina acutifrons, Oithona plumifera, Oncaea venusta, O. similis, Subeucalanus subcrassus, Temora turbinata, and T. styllifera as the most abundant in his study. The latter also indicated that there are changes in the copepod assembly structure between the upwelling and the non-upwelling periods. He considered Copilia mirabilis, Aetideus acutus, and A. giesbrechti to be indicators of the upwelling period.
Different authors (Expósito, 1997; González, 2003; Márquez-Rojas and Zoppi, 2017; Colina, 2019) have mentioned Acartia tonsa and Temora turbinata as the most important species in terms of numerical representation and frequency within this bay. It is important to point out that these species belong to surface waters or those close to continental shelves and are typical of tropical latitudes. This agrees with the precedents regarding the copepod fauna in the southeastern Caribbean (Michel and Foyo, 1976) and the south of the Gulf of Mexico (Campos-Hernández and Suarez-Morales, 1994; Ruiz-Pineda et al., 2016). This coincidence might be related with these species’ geographical conformation; BM is a semi-closed coastal system with a wide mouth towards the North, reaching 60 m in depth, which decreases progressively towards the South, with depths between 10 and 20 m. It has a great marine influence due to the penetration of sub-surface waters from both the Basin and the Gulf of Cariaco (Okuda et al., 1968; Quintero et al., 2004), even allowing for the entry of species with neritic-oceanic affinity.
Most of these micro-crustaceans make up the most abundant group, representing 40-60% of the zooplankton community (Expósito, 1997; González, 2003; Márquez-Rojas et al., 2007, 2008; Marcano et al., 2010; Narváez et al., 2019). The maximum abundance values often occur during times with the most intense coastal upwelling (González, 2003; Márquez-Rojas et al., 2007, 2008; Brito 2013).
Colina (2019) studied the family Temoridae, identifying Temora turbinata and T. stylifera. The former was more abundant and dominant, which agrees with the works by Kiørboe (2006) and Rimoldi (2008). This is due to its short lifecycle and the high number of generations per year, which grants it a reproductive advantage over the other species.
For this study area, Expósito (1997) provides the first reports of Centraugaptilus rattrayi, Oithona brevicornis, Triconia furcula, and Dioithona oculata in Venezuela (Table 2). The first of these species is mesopelagic, and it was reported during times of upwelling. It has been reported for the Gulf of Mexico and the Caribbean Sea by Owre and Foyo (1967) and Suárez-Morales and Gasca (1998). Oithona brevicornis is epipelagic, marine, and brackish. This agrees with the fact that the specimens were captured in stations close to the oxidation lagoon of the town of Mochima, where salinity values oscillate between 15 and 20 UPS.
As for Triconia furcula, there is some confusion regarding its identification; it may actually be Triconia conifera, since some identification keys mention Triconia conifera var. furcula. Unfortunately, this species was identified in a thesis and was likely not confirmed by a taxonomist, which tends to indicate an erroneous identification.
Dioithona oculata is an epipelagic species from the Indo-Pacific, which might have gone into the Atlantic via the Agulhas current (Razouls et al., 2023). According to Björnberg (1963), this species can be easily recognized for its ocular lenses. It has been recorded in the warmer waters of Brazil in the South Equatorial Current, the Colombian Caribbean, the Gulf of Mexico, Jamaica, Belice, and the Caribbean Sea (Razouls et al., 2023).
González (2003) would later report Euchaeta spinosa and Microcalanus pygmaeus, epi-mesopelagic species, during upwelling at a station located in the bay mouth. The former was also captured by Owre and Foyo (1967) in the Florida current between 50 and 907 m. Microcalanus pygmaeus was also reported by Medellín-Mora and Navas (2010) during upwelling in the Colombian Caribbean.
Centropages typicus is epipelagic, neritic, eurythermal, and one of the most abundant species in the northern Atlantic (Durbin and Kane, 2007). It has been found between Cabo Cape and the Chesapeake Bay, as well as in the Gulf of Maine (Razouls et al., 2023). It may be necessary to conduct a new review or verify this species, as it was reported in an undergraduate thesis (Brito, 2013).
Cariaco basin (CC)
This work mentions 139 species for this basin (Table 2). The maximum sampled depth is 500 m. The first researcher to conduct copepod studies in this basin was Legaré (1961), who identified 64 species and concluded that the deep-water community was characterized by the presence of Euaetideus, Haloptilus, Lucicutia, Rhincalanus, and Scolecithrix. Zoppi (1961) conducted horizontal and vertical samplings (0-500 m) at the eastern end of the Cariaco basin, identifying 31 copepod species. She found Eucalanus monachus, Lucicuta clausi, Oncaea conifera, and Rhincalanus cornutus to be the most abundant in the deeper waters (500-300 m), Clausocalanus furcatus and Calanus minor in intermediate waters (300-100 m), and C. arcuicornis, T. turbinata, and Oithona atlantica in shallower waters (100-50 m).
Later, Legaré (1964) conducted vertical captures from 500 m towards the surface, identifying 102 species. Out of the total capture, 62% was composed of the genera Clausocalanus, Paracalanus, Oithona, and Temora. He concluded that the copepod fauna of the basin consists of a mixture of littoral, oceanic, epipelagic, and mesopelagic species. Furthermore, he indicated that all species are marine, with the vast majority being exclusively tropical.
Cervigón and Marcano (1965) conducted a study on copepods along the northern coast of the state of Sucre, covering the Cariaco basin, and identified 99 species. They observed peak abundance during daylight hours in the intermediate layers (50-100 m); below 150 m, copepods were sparse. Among the most abundant species were Eucalanus pileatus, Clausocalanus arcuicornis, C. furcatus, T. turbinata, Oncea venusta, Microsetella norvegica, and M. rosea. In this study, graphs of the most important species were elaborated, indicating that Nannocalanus minor is typical of the surface layer (< 50 m) and warm waters. Eucalanus pileatus was the characteristic and constant species of the community during the months with prevailing winds and lower temperatures. Paracalanus aculeatus and C. arcuicornis are associated with the surface strata and the abundance of phytoplankton. Mecynocera clausi is rare on the surface. It appears to be connected to the intermediate layers during calm periods and higher temperatures. Euchaeta paraconcinna is typical of the surface layers and the windy and cooler period. Scolecitrichopsis ctenopus and Scolecithricella ctenopus, typical of this basin at 100 m, rarely reach 50 m, only doing so in times with cooler water (< 24 °C). Temora turbinata and T. stylifera are abundant in the surface layers, and, below 100 m, their abundance drastically decreases. They are associated with calm periods and high temperatures, while Temoropia mayumbaensis is typical of the intermediate layers - even below 100 m, it is relatively abundant. Lucicutia flavicornis was never captured on the surface during daylight hours and was seen to be abundant from 50 m onward. Haloptilus longicornis is one of the most typical species of the intermediate and deep layers of the basin. Oncaea venusta was never found on the surface, despite being one of the most abundant species throughout the year - its abundance increases when the water temperature is warmer. Farranula gracilis is one of the most evident indicator species, clearly linked to the surface layers and high temperatures (>27 °C).
Pineda-Polo (1979) identified a new copepod species from the family Euaugaptilidae originating in the subsurface waters (110 m) of the basin. He provided significant morphological descriptions that distinguish this species from other members of the genus. He proposed the name Euaugaptilus fosaii.
Márquez-Rojas et al. (2009) studied the zooplankton in the surface layer (< 100 m) of the CC. Copepods turned out to be the most abundant group (> 60% of the total abundance during all sampled months). They identified 40 species. The most abundant and dominant were P. quasimodo, T. turbinata, Undinula vulgaris, P. aculeatus, Calanopia americana, and C. arcuicornis, which show similarities with the warm tropical waters that occupy the region, as confirmed by Björnberg (1981) and Suárez-Morales (1997). These authors also identified the Atlantic oceanic forms Euchaeta marina, T. stylifera, C. furcatus, and C. pavo, as indicated by Owre and Foyo (1967). The presence of these specimens in the basin could be explained by the effect of coastal upwelling, in addition to the open connection of the waters in this basin with the neighboring waters of the Atlantic Ocean, possibly being dragged into Caribbean Sea waters due to eddies or currents (Astor et al., 2003, 2004). The authors confirmed that these microcrustaceans are important bioindicators of the coastal upwelling phenomenon typical of the region (Rueda-Roa and Müller-Karger, 2013)<.
Among the studies conducted in this basin which expanded the knowledge of the group by constituting new records for Venezuelan waters, the works by Legaré (1964) and Cervigón and Marcano (1965) stand out. They identified the presence of Gaetanus miles, G. minor, Euchaeta media, Calocalanus pavoninus, Lophothrix latipes, Spinocalanus abyssalis, Parundinella spinodenticula, Sapphirina sinuicauda, Vettoria granulosa, Oculosetella gracilis, and Corycaeus crassiusculus. Meanwhile, for the Caribbean Sea, Cervigón and Marcano (1965) and Márquez-Rojas et al. (2009) provided the first reports of the presence of Euchirella formosa and Paraeuchaeta tonsa, respectively (Table 2). Euchirella formosa is an epipelagic species, which was found between 100 and 115 m deep at station 3 (located between Tortuga Island and Blanquilla, Venezuela). The closest area where this species has been found is southern Brazil. On the other hand, P. tonsa was captured between 75 and 100 m deep during the upwelling season, and it has been reported for the Caribbean coast of Costa Rica (Morales-Ramírez and Suárez-Morales, 2008) and the Colombian Caribbean (Medellín-Mora and Navas, 2010; Gaviria et al., 2019).
Gulf of Cariaco (GC)
Recently, Márquez-Rojas et al. (2020) elaborated an inventory of the planktonic copepods recorded for this gulf, reporting a total of 136 species, out of which 71 belong to the order Calanoida (52,2%), 48 to Cyclopoida (35,3%), 10 to Harpacticoida (7,3%), and 7 to Siphonostomatoida (5,1%) (Table 2). At the family and genus levels, the order Calanoida is notably the most diverse group, with 18 families and 38 genera, followed by Cyclopoida with 6 and 14 and by Harpacticoida with 9 and 11. Within Calanoida, the main genera in order of abundance are Temora, Acartia, Paracalanus, and Subeucalanus, and, within them, the species T. turbinata, A. tonsa, and P. quasimodo have been considered to be the dominant copepods in the area (Legaré, 1961; Zoppi, 1961; Márquez-Rojas et al., 2006; Márquez-Rojas, 2010).
In the review carried out by Márquez-Rojas et al. (2020), within the order Cyclopoida, only the family Oithonidae was found, solely with the genus Oithona and seven species. Here, Oithona plumifera and O. setigera were the most abundant and frequent. This is in line with the coastal and estuarine areas of the Gulf of Mexico and the Caribbean Sea (Owre and Foyo, 1967; Suárez-Morales and Gasca, 1997). All Oithona species documented in this research have been reported for the Mexican Caribbean (Suárez-Morales and Gasca, 1998), the Caribbean Sea (Owre and Foyo, 1967), and in the database presented by Razouls et al. (2023) for Venezuela, the Caribbean, and Florida.
In the inventory by Márquez-Rojas et al. (2020), concerning the suborder Poecilostomatoida, the family Corycaeidae was the most abundant, with five genera and 22 species. For the Venezuelan Caribbean region, only 23 Corycaeus species and 3 Farranula species have been recorded (Legaré, 1964; Zoppi, 1961; Cervigón and Marcano, 1965; Razouls et al., 2023). Of these, 19 have been reported for the Gulf of Cariaco. The most common and abundant for the southeastern Caribbean region and the Cariaco basin and gulf are Corycaeus speciosus, Urocorycaeus lautus, and Ditrichocorycaeus amazonicus (Legaré, 1964; Cervigón, 1964; Cervigón and Marcano, 1965; Márquez-Rojas et al., 2014a, 2014b).
Within this suborder, the families Oncaeidae and Sapphirinidae are also representative, with seven and ten species respectively. Oncaea mediterranea is the most abundant and frequent, followed by O. media and O. venusta. Among the Saphirinidae, Copilia mirabilis, Sapphirina angusta, S. intestinata, S. opalina, and S. nigromaculata are the most common and abundant species.
Harpacticoids are typically benthic but include a few planktonic species. Euterpina acutifrons is the most frequent harpacticoid in plankton samples in this gulf, as well as in the Gulf of Mexico and the Caribbean Sea (Legaré, 1961; Zoppi, 1961; Márquez-Rojas et al., 2006; Hernández-Trujillo et al., 2010). Microsetella rosea, Macrosetella gracilis, and Miracia efferata were also identified. Serrano (2015), in his study in the Turpialito bay, identified 11 harpacticoid species, including the first reported case of Porcellidium fimbriatum in the gulf.
The harpacticoid P. fimbriatum has been not reported for Caribbean Sea waters but has been well-studied in Australian waters (Harris and Robertson, 1994) and the Mediterranean Sea (Harris, 2014). This species requires taxonomic confirmation, as it was reported in a thesis.
Siphonostomatoid copepods of the genus Caligus are among the most diverse representatives of crustacean parasites or ectoparasites of teleost fishes. These parasites, in their initial life stage, are free-swimming and part of the zooplankton. Lepeophtheirus nordmanni and Cecrops latreillii were first found as ectoparasites of the sunfish Mola mola in the Gulf of Cariaco (Díaz-Díaz, 2000). Later, Kim et al. (2019) reported Caligus littoralis, C. evelynae, C. praetextus, and C. rufimaculatus for the first time in the Gulf of Cariaco, indicating that C. evelynae and C. rufimaculatus had only been found in plankton and that their hosts were still unknown. Furthermore, they revealed that these had not been previously recorded in Venezuelan waters or the Venezuelan Caribbean, so their discovery in the study area contributes to the zoogeographic knowledge of their regional distribution.
Northeastern Caribbean Sea (MCN)
One of the first and most extensive studies in terms of both duration and distance was carried out by Cervigón and Marcano (1965), who studied zooplankton from La Tortuga Island to the Venezuelan Atlantic coast, including the Cariaco Basin, the coasts of the Nueva Esparta state, the Gulf of Paria, and Boca de Serpiente. They conducted samplings at 38 stations throughout this vast northeastern area of the country from 1962 to 1965, using standard zooplankton nets and the Clarke-Bumpus net, aiming to understand species diversity. They identified 172 species, out of which 35 belonged to offshore stations (12), corresponding in this study to the MCN area (11°11’ North, 63°52’ West; Fig. 1).
Among the offshore stations, the one they named Station 6 (11°55’ North, 64°37’ West) recorded the highest number of species (14): Eucalanus elongatus, Aetideopsis multiserrata, Scottocalanus helenae, Metridia brevicauda, M. princeps, Lucicutia magna, L. ovalis, Heterorhabdus abyssalis, Paraheterorhabdus vipera, H. longicirrus, Euaugaptilus nodifrons, E. palumbii, Nullosetigera bidentata, and Conaea rapax. Samplings at this station were conducted from a 1000 m depth to the surface.
Station 3 also showed a high number of mesopelagic species. It is situated between La Tortuga Island and La Blanquilla Island. The copepods identified include Spinocalanus aspinosus, Aetideus bradyi, Pseudeuchaeta brevicauda, Phaenna spinifera, Archescolecithrix auropecten, H. longiceps, and Sapphirina scarlata. It is worth highlighting that Aetideus bradyi was captured at this station, which corresponds to the first record of this species for Venezuela. According to Razouls et al. (2023), it is epi- and upper mesopelagic, with capture records above 200 m deep. Its biogeographic distribution is said to include southern Brazil, the Gulf of Guinea, northwest Africa, and Cape Verde Island. Therefore, its presence in Venezuela could be due to the upwelling waters off the coast, as previously indicated, thereby extending its geographical distribution in the Caribbean Sea.
Zoppi (1977) collected zooplankton samples on the continental shelf extending from the Araya peninsula in the west (10° 38’ N, 64° 18’ W) to the Paria peninsula in the east (10° 43’ N, 61° 52’ W), the southern coast of Margarita Island (10° 59’ N, 64° 25” W) up to the parallel 11° N and the meridian 61° W, forming a rectangle (Fig. 1). She identified 44 copepod species, with the first records of Oithona nana and Paracalanus crassirostris in Venezuelan waters. The highest abundances were observed in the stations between Carúpano and Araya and in Stations 5 (10° 42’ N, 61° 52’ W) and 9 (11° 05’ N, 63° 20’ W). She also mentioned that 14 species were the most common and abundant.
This area reports the highest number of copepod species (166) (Table 2). Firstly, this could be due to the vastness of the area and, secondly, to the presence of deep-water species in this region - some stations exceed 500 m in depth. This serves as evidence of the upwelling waters advancing from off the coast, which, due to advection phenomena, extend to reach this region. Based on the results obtained for this region, it can be noted that one of the two main upwelling areas of the country lies in eastern Venezuela. This results in increased fertility and fishery production, as the Subsurface Subtropical Water mass is the one that fuels the upwelling during the first months of the year (drought season). In the second half of the year, when the upwelling relaxes, the area is enriched with organic matter from the Orinoco River and the contribution of phytoplanktonic biomass from coastal lagoons, semi-closed gulfs, and bays. Likewise, the breadth of the continental shelf plays a fundamental role in enriching this zone, with the presence of archipelagos and major and minor islands that cause local blooms and the concentration and retention of plankton, enhancing biological productivity and in turn fostering the reproduction of these organisms (Rueda-Roa et al., 2018; Gómez and Acero, 2020).
Paria Gulf and Peninsula (PGP)
Cervigón and Marcano (1965) conducted zooplankton samplings with the aim of understanding species diversity from La Tortuga Island to the Venezuelan Atlantic coast, including the Gulf of Paria and Boca Serpiente. Among the copepods identified in this area are Pleuromamma piseki, Labidocera fluviatilis, L. nerii, Candacia bispinosa, Pontellina plumata, Pontellopsis perspicax, Sapphirina angusta, and S. maculosa.
Zoppi (1977) collected zooplankton samples in the Paria Peninsula and Gulf. She established four stations (Fig. 1) with depths between 20-80 m and in a sector adjacent to the Atlantic Ocean, between 10° 15’ N and 62° 00’ W in its eastern boundary line (Fig. 1). She observed the highest concentrations of copepods between February and April. Generally, they appeared more abundantly in the stations in the Gulf of Paria and at Station 5 (10° 42’ N, 61° 52’ W). The common and most abundant species were T. turbinata, T. stylifera, O. mediterranea, P. attenuatus, S. subtenuis, P. aculeatus, P. parvus, C. arcuicornis, C. furcatus, A. clausi, O. plumifera, O. nana, C. giesbrechti, and A. typicus. Nevertheless, she also noted that T. turbinata and P. parvus were the dominant and frequent species, while P. aculeatus exhibited the highest abundance in said gulf. In addition, she pointed out the presence of specimens indicative of freshwater, such as Pseudodiaptomus acutus, Parvocalanus crassirostris, and A. clausi.
Years later, the Environmental Baseline Project for the Deltana Shelf (LBAPD) was carried out, which was divided into two campaigns: the rainy season (October 25-30, 2004) and the dry season (May 28 to June 9, 2005). It consisted of 57 stations, distributed across major sectors, namely: the Gulf of Paria (Stations 1-6), Boca de Serpiente (Stations 7-13), and the Deltan Continental Shelf (Stations 14-57) (See Fig. 2 in Martín et al., 2007). For the purposes of this study, only the stations in the Gulf of Paria were considered. The copepods were dominant, representing 61.90 % (Martín et al., 2007; Zoppi et al., 2008). In the rainy season, the highest abundances were found in the Gulf of Paria and Boca de Serpiente, unlike the dry season, during which they were grouped in Boca de Serpiente and the oceanic zone.
Márquez-Rojas (2005) studied the temporal and spatial variation of zooplankton in this area within the framework of the Mariscal Sucre Project (LBPMS), funded by Petróleos de Venezuela (PDVSA), in order to gain knowledge of natural resources and quantify potential environmental impacts. This project covered 50 stations, sampled in two expeditions: during the dry season (March 2005) and during the rainy season (October 2005). The distribution of the sampling stations covered two major sectors: the Northeastern part of the northern continental shelf of the Paria Peninsula, with depths ranging from 4 to 111 m (Stations 1-22) and the northern part of the Gulf of Paria from 7 to 57 m deep (Stations 30-50) (see Fig. 1 in Márquez-Rojas, 2005). 61 copepod species were recorded, 57 of which had been reported in other studies in the Western Tropical Atlantic and the South American Caribbean Sea (Calef and Grice, 1967; Owre and Foyo, 1967; Campos-Hernández and Suárez-Morales, 1994). The remaining species provide new contributions to the study area: Pseudodiaptomus acutus, Mesocalanus tenuicornis, Clytemnestra scutellata, and the genus Monstrilla sp. Within the same project, Márquez-Rojas and Marín (2007) conducted an inventory of planktonic calanoid copepods while making some zoogeographic considerations. They identified 42 and 49 species of copepods for the dry and rainy periods, respectively. The entirety of the copepod species in the studied area belong to tropical environments: 33% are typical of oceanic waters, 49% showed neritic affinity, and 18% are common inhabitants in both neritic and oceanic zones, with the dominant presence of epiplankton or subsurface species (65%).
Cova (2018) analyzed the composition and abundance of zooplankton in the North and South sectors of the Paria Peninsula in October 2015, in 16 stations spanning between 11°02’ North, 62°81’ West (Fig. 1). Copepods showed a high frequency of occurrence (51,8%), with 43,7% corresponding to Calanoida and 8,08% to Cyclopoida. In general, Station 10, located in the South region of the peninsula, reported the lowest percentage of copepod occurrence (15,5%), while Station 14, located in the North portion, had the highest percentage (89,4%). 13 copepod families and 29 species were identified. The most representative were Temora turbinata, Centropages velificatus, Acartia lilljeborgi, A. tonsa, Labidocera scotti, Subeucalanus subcrassus, S. crassus, Corycaeus catus, C. speciosus, C. lautus, Farranula gracilis, and F. rostrata. It was also mentioned that Candacia curta, Eucalanus elongatus, Neocalanus gracilis, and Clausocalanus furcatus were the only species absent in the southern zone; while C. furcatus, L. acuta, Undinula vulgaris, Acrocalanus longicornis, Paracalanus quasimodo, Mecynocera clausi, Nannocalanus minor, and Paraeucalanus sewelli were found only in the southern region of the Paria Peninsula.
It is important to note that this region, despite being one of the most significant environments for the country’s fisheries and, in recent years, a focus of the promotion of oil operations in the northeastern region of Venezuela, the compiled information and copepod inventory provided by this research show limited studies on copepod taxonomy. Only 77 species have been identified so far (Table 2). Regarding these species, it is crucial to highlight, only for this region of Venezuela, the presence of Sapphirina maculosa, as reported by Cervigón and Marcano (1965); of Labidocera johnsoni, as mentioned by Cova (2018); and of the genus Monstrilla, according to Márquez-Rojas (2005). Labidocera johnsoni requires taxonomic confirmation, as it has been exclusively reported in unpublished references (i.e., undergraduate thesis). Furthermore, note that this species has been reported for the Eastern Tropical Pacific (Central America, Galápagos, northern Peru) according to Razouls et al. (2023).
FINAL CONSIDERATIONS
Nomenclature changes
The species list (Table 2) was developed based on the scheme published by Márquez-Rojas et al. (2020), with some modifications. In the bibliographic references of Table 2, the species names are indicated as they were written in the original publication. Over time, there have been several changes in the nomenclature of orders, families, genera, and species. Within the order Calanoida, four Eucalanus species (E. crassus, E. subtenuis, E. pileatus, and E. monachus) are now considered to belong to the genus Subeucalanus, and Eucalanus attenuatus now belongs to the genus Pareucalanus (family Eucalanidae). Within this same family, Cervigón and Marcano (1965) located Rhincalanus cornutus, which now corresponds to the family Rhincalanidae.
According to the classification used in this inventory, in the family Paracalanidae, Paracalanus scotti changed its genus to Parvocalanus, and Ischnocalanus plumulosus to Calocalanus. Similarly, Calocalanus contractus, C. pavo, C. pavoninus, C. styliremis, and Mecynocera clausi were relocated to this family. Some members of the family Pseudocalanidae were moved to Clausocalanidae. Some species from the family Aetideidae changed the genus Euaetideus to Aetideus. Within the family Scolecitrichidae, the following species were synonymized: Scolecithricella auropecten as Archescolecithrix auropecten, Scolecithricella echinatus as Scaphocalanus echinatus, and Scolecithricella marquesae as Scolecitrichopsis ctenopus. In the family Augaptilidae, the species Haloptilus longicirrus is currently not accepted and has been synonymized as Haloptilus longicirrus. The calanoid Phyllopus bidentatus originally belonged to the family Arietellidae and was moved to Nullosetigeridae. It was taxonomically rewritten by Soh et al. (1999) as Nullosetigera bidentata.
As the order Poecilostomatoida is now considered to be a part of Cyclopoida (Khodami et al., 2017), the latter now includes seven additional families in Eastern Venezuela: Clausidiidae, Corycaeidae, Cyclopoida, Lubbockiidae, Oithonidae, Oncaeidae, and Sapphirinidae, with 18 genera and 67 species (Tables 1 and 2). Márquez-Rojas et al. (2014a, b) studied the distribution and abundance of the family Corycaeidae in the Gulf of Cariaco, reporting Corycaeus (Monocorycaeus) robustus and Farranula carinata as the first records for the Caribbean Sea and the Gulf of Cariaco, respectively. In these studies, this family was classified within the order Poecilostomatoida and divided into six subgenera. Starting with the inventory by Márquez-Rojas et al. (2020), the new nomenclature has been adopted. Within the family Corycaeidae, four subgenera of Corycaeus (Agetus, Ditrichocorycaeus, Onychocorycaeus, and Urocorycaeus) were elevated to the genus category. Regarding the family Oithonidae, Oithona oculata now belongs to the genus Dioithona. In the family Oncaeidae, the genus Oncaea genus was divided into two: Oncaea and Triconia (Böttger-Schnack et al., 2011). Oncaea minuta is now called Triconia minuta. The species Oncaea notopus is currently not accepted and was synonymized as Oncaea prolata. Based on the inventory by Márquez-Rojas et al. (2020), the two Lubbockia species were excluded from the family Oncaeidae family and placed in Lubbockiidae. Finally, the species Pachos punctatum used to be classified within the family Oncaeidae (Owre & Foyo, 1967), but it is now separated in the family Cyclopoida and considered to be of uncertain placement (incertae sedis).
Within the order Harpacticoida, the family Clytemnestridae became a subfamily, reorganizing the species into the family Peltidiidae. In this family, Clytemnestra rostrata is now called Goniopsyllus rostratus. Within the family Miraciidae, Miracia minor is currently not accepted and was synonymized as Distioculus minor.
Diversity distribution per area
Within the studied regions, BM, CC, and GC are the ones with the most sampling efforts. In these areas, between 30 and 60% of the copepods of the region have been identified, possibly due to their proximity to the coast, which facilitates field operations. However, in the MCN portion, the highest number of copepod species was recorded (71.86%), given the vastness of the area and the presence of deep-water species (>500 m) in this region, indicating the advances of upwelling waters that occur far from the coast and that, due to advection phenomena, extend to reach this region.
PGP is one of the most productive sectors in the tropics and constitutes an essential area for the feeding, reproduction, and growth of numerous commercially important fish species, according to Lasso et al. (2004). This author noted that, despite being one of the most important environments for fisheries and, in recent years, a focus for boosting oil operations, there has been little interest in research and studies on its marine and freshwater biota, as well as on their relationships with oceanographic conditions. This would explain why only 30% of copepod species have been recorded out of the total identified in the five areas reviewed in this study. The minimal sampling effort in this area is possibly influenced by difficult land access to many beaches located on the north coast, as well as by the high dynamics of the waves in this part of the Venezuelan Caribbean, which hinders access to vessels with scientific equipment.
The presence of oceanic species from the Atlantic on the Venezuelan coasts, even in CC and GC, could be related to coastal upwelling and the communication of these water masses with the Atlantic Ocean, resulting in a movement towards the Caribbean Sea due to eddies or currents (Astor et al., 2004). In this regard, Pauluhn and Chao (1999) and Astor et al. (2004) indicated that eddies flowing northwest with the Caribbean current have been observed in the Caribbean Sea. Sometimes, these eddies are close to the Venezuelan continental shelf, and their true origin is unknown. Certaine gyres form outside the Caribbean Sea, detaching from the retroflection area of the North Brazil current, while others spread from the Caribbean current. This might therefore cause Caribbean waters to penetrate the basin over the passage of La Tortuga channel (Astor et al., 2003). Consequently, it would be feasible to find copepods typical of the Atlantic in the waters of northeastern Venezuela. This was demonstrated with the presence of Euchaeta tonsa and E. spinosa, which have been considered by Owre and Foyo (1967), Bjornberg (1981), and Campos-Hernández and Suárez-Morales (1994) as deep-water species of the western North Atlantic.
Records for the northeastern Caribbean and the Venezuelan Atlantic
In the updated list (Table 2 ), there is a total of 229 reliably identified copepod species, which belong to 5 orders, 41 families, and 97 genera (Table 3). A group of 5 copepod species does not have confirmed identification, as they were mentioned exclusively in undergraduate theses (Triconea furcula, Centropages typicus, Porcellidium fimbriatum, and Labidocera johnsoni).
The total number of species in the northeastern Caribbean and the Venezuelan Atlantic represents slightly less than 50% of the known copepod diversity in the Greater Caribbean. For the Caribbean Sea, 468 species are reported (Medellín-Mora and Navas, 2010) , and, for the southeastern sector, which is part of the Colombian Caribbean, 214 species have been recorded (Gaviria et al., 2019) , which recently increased to 247 (Dorado-Roncancio et al., 2021) . The number of species recorded in this inventory is similar to that in other Caribbean areas, e.g., in Mexican waters, the total known species number is 223 (Suárez-Morales and Gasca, 1998; Hernández-Trujillo and Esqueda-Escárcega, 2002) .
In the northeastern Caribbean and the Venezuelan Atlantic, calanoid copepods (142 species) make up the order with the highest diversity. This inventory indicates the species that were once mentioned as contributing to the expansion of their distribution for Venezuela and the Caribbean Sea: Aetideus bradyi, Euchirella formosa, Eucalanus elongatus, Pontella mediterranea, Scolecitrichopsis tenuipes, and Paraeuchaeta tonsa.
Within the inventory of calanoids, Paracalanus pygmaeus was included, which was identified by Cervigón and Marcano (1965) and Caraballo (1976) for the Cariaco Basin as well as on the coasts of the state of Sucre. These authors used the keys of Tanaka (1956) and Vervoort (1963, 1965) to identify this specimen. However, according to WoRMS (2023), its status is uncertain, as there is taxonomic or nomenclatural uncertainty, and it cannot be classified as accepted or not accepted. Meanwhile, in Razouls et al. (2023), there are conflicting opinions that P. pygmaeus and P. denudatus are conspecific. Therefore, a more exhaustive review of this species is anticipated.
The order with the second-highest number of species (67) is Cyclopoida (including Poecilostomatoida). The genera with the most species are Sapphirina (12 species), Oithona (9), and Oncaea (6). The family Corycaeidae, which is well represented (8 genera and 25 species), has already been recorded (Márquez-Rojas et al. 2014 a,b; 2020). The species Oncaea venusta venella, Sapphirina maculosa, and Ditrichocorycaeus andrewsi constituted new records for northeastern Venezuela in the works by Legaré (1964) , Cervigón and Marcano (1965) , and Morales (2008) .
In our list, 13 species of the order Harpacticoida were compiled. This is a low number compared to the marine harpacticoid species. Suárez-Morales et al. (2006) recorded 178 species in the Caribbean Sea, while Gómez and Morales-Serna (2014) listed 71 in Mexico. The 13 harpacticoid species recorded for northeastern Venezuela represent only 7%, indicating a significant knowledge deficit of this order in the region.
Due to their rarity in plankton and taxonomic complexity, there are large geographical areas where the fauna of monstrilloid copepods remains largely unknown (Suárez-Morales, 2015; Suárez-Morales & Castellanos-Osorio, 2019) . In this region, the order Monstrilloida is represented by one genus: Monstrilla. This record corresponds to an adult male found in plankton samples from the PGP sector (Márquez-Rojas, 2005) . In the Caribbean and the Gulf of Mexico, this order is represented by 24 species (Suárez-Morales, 2015), so an increase in the number of species for northeastern Venezuela is expected in the future.
Siphonostomatoida is a diverse order; all species are parasites or are associated with other species of vertebrates and invertebrates (Hernández-Trujillo, 2015). Within this order, the family Caligidae is the richest in species (Kim et al., 2019) . Caligidae contains 559 species in two genera: Lepeophtheirus (162 species) and Caligus (268 species) (Ho and Lin, 2004). As of 2003, the known Venezuelan caligid fauna consisted of 10 Caligus species (Ho and Bashirullah, 1977; Díaz-Díaz, 2000; Zambrano et al., 2003) . Suárez-Morales et al. (2012a, 2012b) would later increase the number to 12. Subsequently, Kim et al. (2019) expanded it to 13 with the description of Caligus littoralis in the waters of the Gulf of Cariaco and the 60th species of the genus recorded in the Neotropical region. Out of the total Caligus species for Venezuela, five have been reported for the Gulf of Cariaco in this inventory, in addition to the species Lepeophtheirus nordmanni and Cecrops latreillii.
It is worth highlight the importance of reviewing and verifying the specimens that were only classified at the genus level: Bradyidius, Xanthocalanus, Tigriopus, Heterolaophonte, Parategastes, and Monstrilla. This might be due to the lack of bibliographic support at the time of identification or to the fact that only one specimen was captured.











 text in
text in