Introduction
The pigments used in various industries, such as food, cosmetics, and pharmaceuticals, play a crucial role in providing products with a wide range of colors and beneficial health properties, significantly influencing consumer acceptance 1. Depending on their origin, these pigments are classified into two main groups: synthetic and natural.
Synthetic dyes, which are artificially produced for a long time, present disadvantages due to safety and health concerns. Although widely used compared to natural dyes, synthetic dyes may pose health risks due to their artificial origin and the presence of harmful chemical additives 2. Significant health risks include diseases such as cancer, migraines, allergic reactions, and kidney and thyroid problems, as well as negative effects on behavior and overall health. Their toxicity can lead to their accumulation in organs and body systems, underscoring the importance of avoiding their use in favor of safer natural alternatives 2.
On the other hand, pigments of natural or biological origin, such as carotenoids and phycocyanins, offer a range of significant benefits in the food, cosmetics, and pharmaceutical industries. These natural pigments contain phytonutrients with antioxidant and anti-inflammatory properties that are beneficial to human health and enhance immune system activity 3,4. Additionally, natural pigments are an ethical and sustainable alternative to synthetic dyes because they are derived from renewable resources and are thus environmentally friendly 4,5.
Phycocyanin, a photosynthetic pigment present in cyanobacteria and red algae, is utilized in the cosmetic and nutraceutical industries due to its fluorescent and antioxidant properties 5. Carotenoids, another type of photosynthetic pigment, are responsible for the yellow, red, and blue coloring of microalgae and cyanobacteria and possess antioxidant and protective properties 6.
Microalgae and cyanobacteria, photoautotrophic microorganisms and prokaryotes capable of harnessing renewable resources such as sunlight, inorganic salts, and CO2 to synthesize proteins, carbohydrates, lipids, and pigments serve as a significant source of metabolites for various industries 6. However, the production of these metabolites presents challenges requiring in-depth knowledge of microorganism cultivation and product recovery technologies 5,7.
Heterotrophic cultivation using microalgae and heterotrophic cyanobacteria such as Scenedesmus and Arthrospira offers significant advantages over conventional autotrophic methodologies. By overcoming challenges related to CO2 supply, light requirements, and reduced exposure to contamination and physical space constraints, it becomes more applicable to industry. Hu et al. (2017) 8 indicate that this method has been increasingly considered for commercial applications. This is attributed, among other factors, to the increased potential for carotenoids, pigments with active antioxidant properties in demand in the health supplements industry. Additionally, Flórez-Miranda et al. (2017) 9 have demonstrated that lutein production in Scenedesmus can be enhanced through manipulation of cultivation conditions, such as nitrogen sources and temperature, supporting a biphasic culture now termed heterotrophic and photosynthetically induced. Nutrient variability and photoperiod optimizations are crucial for maximizing the synthesis of pigments such as phycobiliproteins and carotenoids; this represents an ideal case study for future research delving into these factorial variables, as proposed for improving pigment yields in cyanobacteria. Pagels et al. (2020) 10 emphasize unlocking the potential of these microorganisms for industrial pigment production through bioprospecting and heterotrophic cultivation techniques, focusing on elucidating and enhancing metabolic pathways and cultivation techniques.
The essential nutrients for the production of photosynthetic pigments in cyanobacteria and microalgae include carbon, nitrogen, and phosphorus 11. These nutrients play a crucial role in the metabolism and growth of these photosynthetic organisms and are essential for pigment synthesis, which is vital for light capture and photosynthesis. Carbon is utilized in the synthesis of organic molecules such as chlorophyll and carotenoid pigments, which are vital for light capture and photosynthesis. Carbon deficiency can affect pigment production and thus the capacity for photosynthesis 11-12. Nitrogen is an essential nutrient for the growth of cyanobacteria and microalgae. When the dissolved nitrogen concentration in water is low, cyanobacteria can accumulate nitrogen in a polypeptide compound called cyanophycin, which serves as a reserve for atmospherically fixed nitrogen. Additionally, the accessory photosynthetic pigment phycocyanin can also serve as a nitrogen source for cyanobacteria. Nitrogen is an important component of chlorophyll molecules, the primary pigment in photosynthesis. Nitrogen deficiency can reduce chlorophyll production and thus affect photosynthetic capacity. When dissolved nitrogen concentrations are low, cyanobacteria first degrade cyanophycin and then phycocyanin to obtain nitrogen 11.
Phosphorus is often limiting in aquatic environments, and its incorporation is essential for energy metabolism and the production of cellular compounds such as membranes and DNA. Phosphorus is essential for the production of ATP, which is an energy molecule necessary for photosynthesis. Phosphorus deficiency can affect ATP production and thus photosynthesis capacity. Cyanobacteria and microalgae can optimize the uptake of low and fluctuating nutrient concentrations, allowing them to colonize a wide variety of habitats 12-13. Moreover, environmental conditions directly related to the circadian cycle or light intensity are also crucial for the production of photosynthetic pigments. The photoperiod affects the production of photosynthetic pigments and the efficiency of photosynthesis, which in turn can influence the production of photosynthetic pigments such as phycocyanins and carotenoids 14.
In this context, the bioprospecting of cyanobacteria and microalgae for obtaining pigments of industrial interest and enriched biomass is a relevant research field that explores the biodiversity of these microorganisms in water sources, analyzing production variables to develop new sources of metabolites and generate added value to production chains 15,16.
The present work focuses on bioprospecting new sources of industrial metabolites, especially natural dyes such as phycobiliproteins and carotenoids, from Arthrospira platensis UTEX1926 and Scenedesmus sp. strains. The effects of photoperiod and the C/N/P nutrient ratio on the production of industrially relevant metabolites were investigated with the aim of developing biotechnological byproducts efficiently and profitably in Colombia.
Materials and Methods
The methodology implemented herein is divided into two main sections. The first section pertains to standardized protocols for biomass, carotenoid, and phycocyanin extraction and quantification. This section details the extraction and quantification process of these components, including spectrophotometric determination. The second section describes the cultivation conditions, such as the photoperiod and experimental design, implemented in the research. This section explains the cultivation conditions, including the photoperiod and C/N/P ratios utilized in the various experimental designs implemented in the research.
Protocols
Biomass Quantification:
Biomass was estimated using the dry weight method. At the end of the cultivation period, aliquots of 20 mL of medium were taken, which were then filtered through precombusted Whatman GF/C filters for 1 hour at 100°C. Subsequently, the filters were placed in an oven at 100°C for 1 hour, followed by 12 hours in a desiccator until a constant weight was reached. The biomass amount in grams per liter was determined by the weight difference of the filters 17.
Carotenoid Extraction and Quantification:
The extraction and quantification of pigments were conducted at the end of the cultivation period. Filters previously combusted with biomass underwent mechanical disruption in 15 mL Falcon tubes. Two grams of glass beads (0.5 mm diameter) and 2 mL of phosphate buffer per filter (8 mM Na2HPO4, 2 mM NaH2PO4, 140 mM NaCl, pH 7.4) were added, and the mixture was homogenized by vortexing for 4 minutes. Subsequently, to separate the extracted carotenoids, 3 mL of chloroform was added, and the mixture was centrifuged at 3400 RPM for 8 minutes. This process was repeated until the cells were colorless. Finally, the carotenoid concentration was determined using Equation (1) described by 18, employing pigments carried in chloroform.
Phycocyanin extraction and quantification:
The extraction of phycocyanins was based on the method described by 19. Once the filters contained biomass and were free of moisture, they were suspended in 10 mL of phosphate buffer solution (0.15 M, pH 7.0) along with approximately 2 grams of glass beads (0.5 mm diameter). The solution was mixed using a vortex at maximum speed for 3 rounds of 2 minutes, after which the sample was allowed to rest for 1 minute in an ice bath. At the end of the process, the sample was stored in a refrigerator at 4°C for 21 hours. To separate the cells from the extract, centrifugation was performed at 3400 RPM for 20 minutes. The supernatant (blue in color) was collected and measured in a spectrophotometer at 620, 652, 562, and 280 nm. The concentrations of phycocyanins (C-PC), allophycocyanins (APC), and phycoerythrins (PE) were calculated using Equations (2), (3), and (4), respectively 20.
Microorganisms and Conditions, strain Conditions and Maintenance
Strain Conditions and Maintenance Arthrospira platensis UTEX1926 and Scenedesmus sp. were obtained from the INNovalgae laboratory at the Francisco de Paula Santander University, located in the municipality of Los Patios, Norte de Santander, Colombia. The Scenedesmus sp. strain was isolated from a sample of a municipal thermal spring located in Norte de Santander, Colombia. Arthrospira platensis UTEX1926 was cultured in Zarrouk's medium 21, and Scenedesmus sp. was cultured in Bold Basal medium 22 under a 12:12 (light:dark) photoperiod at a temperature of 27°C. Every 25 days, the strains were inoculated into Petri dishes and liquid media using cylindrical reactors with a diameter of 4 cm and a height of 11 cm at a working volume of 80% and a pre-filtered air flow rate of 0.6 vvm.
Photoperiod Evaluation
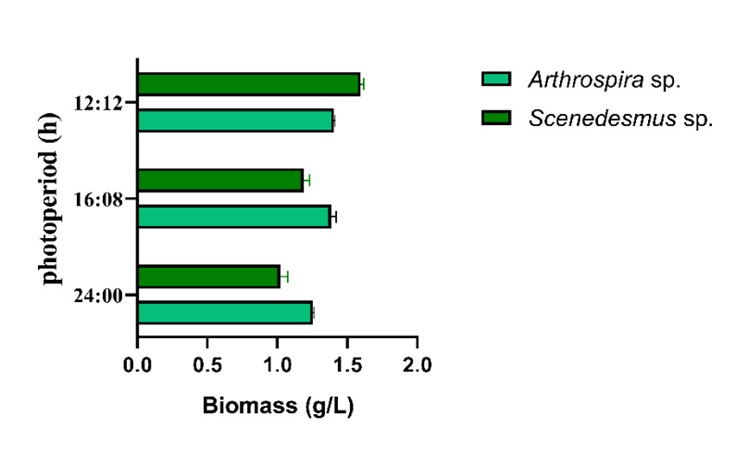
The analysis was conducted by cultivating Arthrospira sp. and Scenedesmus sp. strains in 500 mL reactors with a working volume of 250 mL of previously sterilized liquid medium defined for each microorganism. Each reactor was connected to a prefiltered air injection system (0.6 vvm) and subjected to different photoperiods (12:12; 16:08; 24:00 light:dark) with a radiation of 100 μmol m-2 s-1 for 20 days. At the end of the cultivation period, the concentration of produced biomass, as well as total carotenoids and phycocyanin, was evaluated.
Determination of the effects of carbon, nitrogen, and phosphorus sources
To determine the effect of the concentration of carbon (sodium bicarbonate), nitrogen (sodium nitrate), and phosphorus (potassium phosphate) sources, a nonfactorial Plackett‒Burman experimental design coupled with central points was used in STATISTICA 7.0 software 23. Table 1 shows the established C/N/P ratios for experimental designs for both Arthrospira platensis UTEX1926 and Scenedesmus sp. concerning phycocyanin and carotenoids, respectively.
Table 1 C/N/P levels for Arthrospira platensis UTEX1926 and Scenedesmus sp.
| Strain | NaHCO3 (g/L) | NaNO3 (g/L) | K2HPO4 (g/L) | ||||
|---|---|---|---|---|---|---|---|
| Low level | High level | Low level | High level | Low level | High level | ||
| Arthrospira platensis UTEX1926 | 1.36 | 18.36 | 0.63 | 2.3 | 0.1 | 0.63 | |
| Scenedesmus sp. | 0.13 | 0.46 | 2.65 | 9.34 | 2.65 | 9.34 | |
Each experiment was performed in 500 mL reactors with a working volume of 50% and an aeration of 0.6 vvm. Additionally, the best photoperiod found in the previous stage (16:8 for Arthrospira platensis UTEX1926 and 12:12 for Scenedesmus sp.) was employed, with a radiation of 100 μmol/m-2 s-1 for 20 days.
Results and discussion
Photoperiod evaluation
Biomass production
The photoperiod, defined as the duration of the light period in a daily cycle, plays a crucial role in biomass production in microalgae and cyanobacteria. Numerous studies have shown that both the quantity and quality of light to which these organisms are exposed can significantly influence their growth and biomass production. For example, it has been observed that different photoperiod regimes, ranging from seconds to continuous light cycles, markedly affect photosynthetic efficiency and cellular composition in biofilms of microalgae such as Nannochloris oculata and Chlorella sp., optimizing biomass production and protein synthesis 24.
Figure 1 illustrates how light stress generated by LEDs can affect the biomass production efficiency of Arthrospira platensis UTEX1926 and Scenedesmus sp., with ranges varying between 4-15% and 30-45% of the total weight, respectively. These findings are supported by previous research, such as that of Niangoran et al. (2021) 25, which indicates that Arthrospira platensis UTEX1926 does not tolerate prolonged light exposure. Additionally, it has been observed that a photoperiod of 12 hours of light and 12 hours of darkness (12:12) mainly favors increased biomass production (Vendruscolo et al., 2019) 26
Similarly, according to 27, which corroborates the importance of light requirements as crucial parameters for crop development, optimal production is evident under a 12:12-hour photoperiod (light:darkness). This observation is attributed to the adequate distribution of light over the specified time, primarily favoring cell division of the strain 28.
Pigment Production
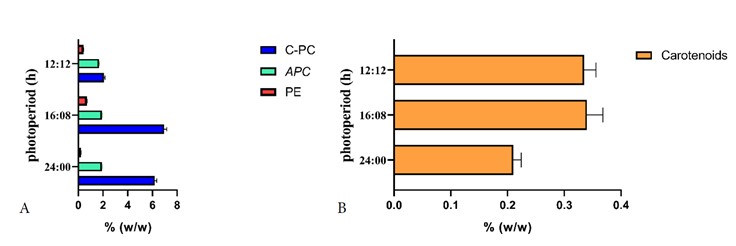
The photoperiod, or light/dark cycle, exerts a significant impact on biomass production and pigment content in microalgae and cyanobacteria. Changes in the relative amount of light greatly affect metabolite production and strain photoacclimation. This occurs through a mechanism involving changes in circadian rhythms, which biochemically affect the conversion of light into products 29.
Figure 2A shows a high content of C-phycocyanin in Arthrospira platensis UTEX1926 (C-PC) under a 16:8 light/dark photoperiod, consistent with the findings of another study 30. This photoperiod appears to be most relevant for C-PC production. In contrast to the biomass production results, where the 12:12 photoperiod had the greatest effect on biomass, this photoperiod resulted in a 66% lower production of phycocyanins than did the 16:8-hour photoperiod. In line with these findings, a study by 31 investigated the effect of different photoperiods (8:16, 10:14, 12:12, 14:10, and 16:8 light/dark) on biomass production and phycobiliprotein content in the cyanobacterium Anabaena variabilis. They found that longer light periods favored growth and phycobiliprotein synthesis, with the 16:8 photoperiod producing the highest C-PC content.
On the other hand, a review conducted by 5 underscores the importance of optimizing light regimes to enhance microalgae biomass production and product formation. The optimal photoperiod may vary depending on the species and desired product. For instance, while longer light periods may increase overall growth and productivity, shorter light periods or even total darkness can trigger the accumulation of certain high-value compounds, such as carotenoids and polyunsaturated fatty acids, in some species 32.
Figure 2B shows a high carotenoid content under the 12:12-hour photoperiod in Scenedesmus sp. This is primarily due to the susceptibility of carotenoid structure to alterations caused by intense light action and processing conditions, allowing for carotenoid degradation under periods of high light stress 33. Consistent with these results, a study conducted by 34, investigating the impact of different light/dark cycles on the growth and pigment content of Chaetoceros sp., revealed that a 12:12 photoperiod yielded the highest contents of chlorophyll-a and carotenoids, while a 24:0 photoperiod (continuous light) resulted in the lowest pigment concentrations. This suggests that the optimal photoperiod for pigment production may differ from that for biomass production 35
Evaluation of the C/N/P concentration
Carbon (C), nitrogen (N), and phosphorus (P) are pivotal elements in biomass production and the deposition of industrially relevant pigments such as phycocyanins and carotenoids. The arrangement of these elements significantly influences industrial pigment production, given their involvement in the molecular formulas of these compounds 34,36.
For instance, the concentration of these nutrients plays a fundamental role in shaping pigment production in microalgae and cyanobacteria. Carbon serves as the primary component of organic molecules, including pigments, while nitrogen is essential for protein synthesis and overall cellular metabolism. Phosphorus, on the other hand, is critical for energy transfer and cellular signaling processes 12-13.
Optimizing the balance of these nutrients is essential for maximizing pigment yield. An imbalance in the C/N/P ratio can disrupt both growth and pigment accumulation, thereby impacting the overall productivity of pigment-producing organisms 13.
Phycocyanin
To determine the most significant experimental conditions for phycocyanin production, a Pareto diagram (Figure 3) was generated. This diagram illustrates the factors influencing production in order of importance of the manipulated variables from various nutrient sources utilized in the medium. The diagram reveals that no significant effects are observed on the final deposition of phycocyanins in Arthrospira platensis UTEX1926, as none of the factors surpass the threshold, indicating that all exhibit a normal distribution in response to the unknown 37. However, the presented results highlight sodium bicarbonate as the factor with the greatest impact on phycocyanin production. Although not statistically significant, these findings align with those of several other studies 38 reporting that supplementing Arthrospira platensis UTEX1926 cultures with sodium bicarbonate increased biomass production and phycocyanin content compared to those in cultures without bicarbonate addition. The authors attributed this effect to increased carbon availability stimulating cellular growth.
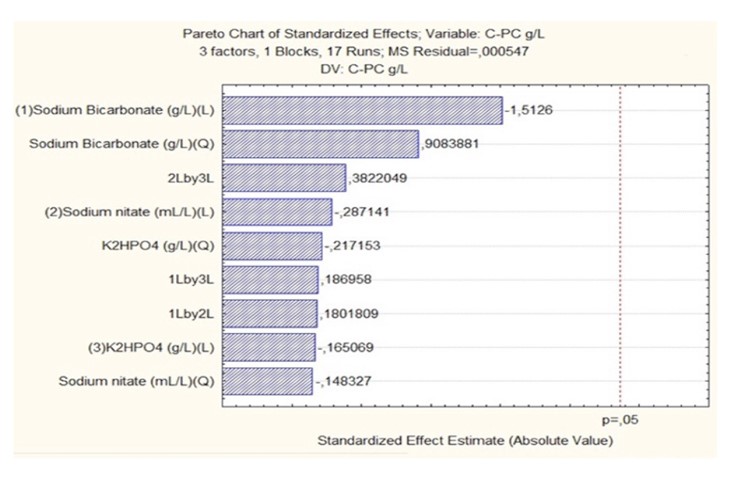
Figure 3 Pareto analysis of the impact of Carbon, Nitrogen, and Phosphorus source factors on final phycocyanin deposition.
Additionally, nitrogen is another key macronutrient and is typically added in the form of sodium nitrate. Adequate nitrogen, including nitrogen-rich phycocyanin, is necessary to support protein synthesis. However, research indicates that nitrogen limitation, rather than excess nitrogen, may enhance phycocyanin accumulation. 40 reported that the phycocyanin content in Arthrospira platensis UTEX1926 increased by 28% under nitrogen-limiting conditions compared with nitrogen-rich media. Nitrogen stress is believed to redirect metabolic flow away from growth and toward nitrogen-containing secondary metabolites such as phycocyanin. However, severe nitrogen deficiency reduces biomass yield, emphasizing the importance of maintaining a balanced C:N ratio.
The response surface methodology employed in this study examined the interactive effects of sodium bicarbonate, potassium phosphate, and sodium nitrate. While sodium bicarbonate exhibited the greatest individual impact, optimal values for maximizing phycocyanin production involve a particular balance of the three macronutrients, as illustrated in Figures 4A-B. These figures depict phycocyanin production as a function of the relationship between sodium bicarbonate and dipotassium phosphate, as well as between sodium bicarbonate and sodium nitrate. Within the studied levels of each variable, maximum production values can be obtained, allowing for the determination of permissible values within the process via desirability criteria.
Moreover, sodium bicarbonate was observed to have a negative effect on phycocyanin production yield through Arthrospira platensis UTEX1926, as reflected by values exceeding 5 g/L. These results align with a study conducted by 39, where both Arthrospira platensis biomass and phycocyanin content decreased when bicarbonate levels exceeded an optimal range due to pH changes and carbon limitations caused by high alkalinity.
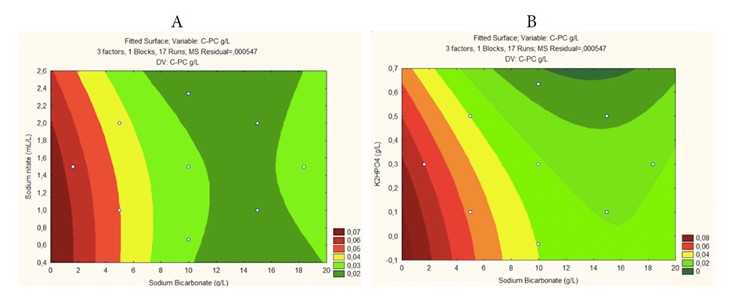
Figure 4 Response surface for final phycocyanin deposition as a function of: (A) Carbon and Phosphorus source; (B) Carbon and Nitrogen.
As shown in Table 2, the levels that maximize phycocyanin accumulation and/or production through Arthrospira platensis UTEX1926 are between 1-2, 0.1-0.3, and 0.5-1 for sodium bicarbonate, sodium nitrate, and dipotassium phosphate, respectively.
Carotenoids
Carotenogenesis is a process involving molecules such as dimethylallyl pyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP), which are compounds of isoprenes primarily composed of phosphorus and carbon. These phosphorus-rich molecules play a crucial role in carotenoid synthesis. As depicted in Figure 5 and according to Lohr M et al. 37, nutrients such as phosphorus and carbon are essential components. This phosphorus (dipotassium phosphate) and carbon (sodium bicarbonate) sources transcend the conventional limits of Pareto analysis, suggesting that they are key variables that directly impact carotenoid production relative to biomass.
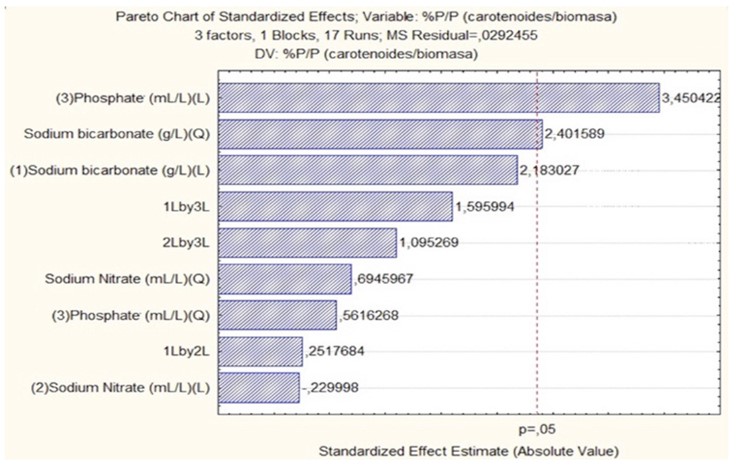
Figure 5 Pareto analysis of the impact of Carbon, Nitrogen, and Phosphorus source factors on carotenoid/biomass ratio
The relationship between phosphorus and carbon availability and carotenoid production may vary depending on the specific cultivation conditions of microalgae. For instance, adjusting carbon and nitrogen concentrations can increase both biomass and total carotenoid concentration, as observed in the study by 12. This implies that optimizing multiple variables, including phosphorus and carbon sources, is crucial for enhancing carotenoid production in microalgae.
However, it is important to consider that the accumulation of metabolites such as carotenoids varies due to differences in the species of microalgae used and their specific metabolic capacities for adaptation. For instance, recent studies have shown that nitrogen availability has a more significant impact on carotenoid accumulation in microalgae than phosphorus levels. Higher carotenoid content was observed under nitrogen-deficient conditions, regardless of the phosphorus concentration in the medium 41-42.
Response surfaces established based on the carotenoid/biomass correlation (Figures 6A and 6B) underscore the importance of variables identified as fundamental in the Pareto analysis (Figure 5): the phosphorus source (dipotassium phosphate) and carbon (sodium bicarbonate). These variables consistently occupy the extremes of their measurements on the response surfaces, suggesting their crucial role in the final pigment deposition. This finding aligns with previous studies emphasizing the importance of phosphorus and carbon in carotenoid production in various species of microalgae 43.
On the other hand, the nitrogen source exhibits dispersed ranges on the response surfaces, with values ranging between the established maximums and minimums. This observation suggests that while nitrogen is an essential nutrient for microalgal growth and carotenoid production, its influence on the carotenoid/biomass ratio is less pronounced than that of phosphorus and carbon. For example, some studies have reported that nitrogen limitation may even promote carotenoid accumulation in certain microalgal species 44, highlighting the variability in adaptation among microalgal species.
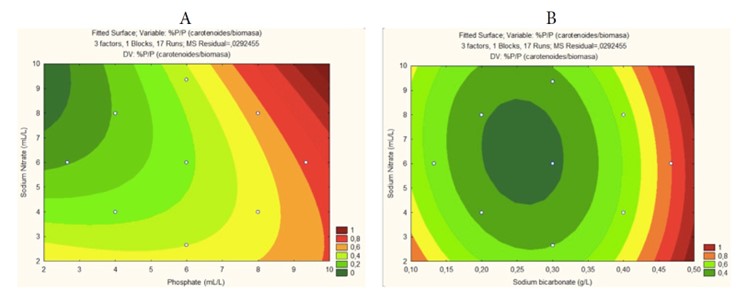
Figure 6 Response Surface for Final Carotenoid/Biomass Ratio as a Function of: (A) Carbon and Nitrogen Source; (B) Phosphorus and Nitrogen
As shown in Table 3, the levels that maximize phycocyanin accumulation and/or production through Arthrospira platensis UTEX1926 are 0.5 - >0.5, 10 - >10, and <2 - 2 for sodium bicarbonate, sodium nitrate, and dipotassium phosphate, respectively.
Conclusions
The use of nonfactorial Plackett-Burman experimental designs facilitated the systematic exploration of independent variables affecting pigment production. This structured experimental approach allowed the identification of influential factors, such as the 16:8 photoperiod and carbon source, for phycocyanin production. Similarly, the 12:12 photoperiod and the phosphate and carbon sources for the carotenoid/biomass ratio play crucial roles in biomass production and pigment content in microalgae and cyanobacteria.
The optimal levels of carbon, nitrogen, and phosphorus significantly influence pigment production in Arthrospira platensis UTEX1926 and Scenedesmus sp. The recommended ranges for optimal phycocyanin production are 1-2 g/L NaHCO3, 0.1-0.3 g/L NaNO3, and 0.5-1 g/L K2HPO4. Conversely, to achieve a greater carotenoid/biomass ratio, elevated levels of NaHCO3 (>0.5 g/L) and K2HPO4 (>10 g/L) and lower levels of NaNO3 (<2 g/L) were found to be beneficial.
While phosphorus-rich isoprene molecules such as DMAPP and IPP are crucial for carotenoid synthesis, it is imperative to adopt a comprehensive approach considering various environmental and nutritional factors. By optimizing cultivation conditions and nutrient proportions, researchers can maximize carotenoid production in microalgae, contributing to sustainable pigment bioproduction practices.
The establishment of optimal conditions for both the final deposition of phycobiliproteins and the pigment-to-biomass ratio (carotenoids/biomass) in Arthrospira platensis UTEX1926 and Scenedesmus sp. demonstrated the potential for enhancing pigment bioproduction. These findings provide valuable insights into refining cultivation strategies to efficiently maximize pigment production.