Introduction
Coffee is a plant belonging to the Rubiaceae family, genus Coffea1. The two most cultivated and harvested varieties are Coffea arabica L. and Coffea canephora, also known as Robusta2.
World coffee production in 2021 was approximately 165.29 million 60 kg bags 3. Brazil is the world's leading producer of coffee, accounting for approximately 40% of global production, followed by Vietnam and Colombia. Colombia is a country recognized worldwide for its high-quality beans 3. In 2021, production of approximately 9.34 million bags of coffee was reported, which represents 5.65% worldwide 3. According to data reported by the Ministry of Agriculture and the National Federation of Coffee Growers of Colombia, in 2020, Caldas accounted for approximately 9.35% of total national production 4,5.
The Castillo variety, an Arabica coffee variety developed by the National Coffee Research Center (CENICAFÉ) in Colombia, has revolutionized the coffee industry thanks to its multiple advantages. Derived from meticulous genetic crossbreeding, this variety stands out for its resistance to coffee rust, a devastating disease for coffee crops in Colombia. This resistance, a product of its genetic diversity, drastically reduces the need for fungicides, thus lowering costs for farmers. Furthermore, the exceptional quality of its coffee, supported by studies from CENICAFÉ's research panel, positions it as a premium option in the international market. With a larger grain size, mostly classified as supreme coffee, the Castillo variety not only opens doors to markets that value this characteristic but also enables producers to fetch better prices for their harvests 6.
In the process of agro-industrial transformation of coffee, a series of residues are generated that are equivalent to 92.4% of the total weight of the fresh fruit 7. These by-products not only cause economic losses, but also generate environmentally unsustainable processes. Some of the main by-products generated in coffee processing are pulp (43.6%), mucilage (14.9%) and wastewater (17.1%) 7,8. It is important to note that the type and amount of waste generated in the coffee transformation process vary according to the method used and the geographical region where the coffee is produced 8-10.
Coffee mucilage is a viscous substance that coats the seeds of the coffee bean and that is removed during the demucilaging or fermentation process of the beans and represents around 14.85% concerning the weight of the raw fruit 8. This secondary product is mainly composed of sugars, pectins and various phenolic compounds, such as gallic acid, chlorogenic acid, ferulic acid, caffeic acid, p-coumaric acid, quercetin, epicatechin and catechin 10,11, these phenols have antioxidant and anti-inflammatory activities 12-14. The above establishes a relevant precedent for the use of coffee mucilage. However, according to studies published by Di Lorenzo et al., and Howitz & Sinclair 15,16, these phenolic compounds can lose their properties when exposed to the environment, where they are subject to factors such as temperature, humidity, oxygen, among others.
Another food matrix of great importance for Colombia and of interest for research is the plantain (Musa paradisiaca), which is a herbaceous plant with a short rhizome. This fruit is positioned as one of the most important crops after rice and wheat. The varieties of plantain in Colombia are Dominican-Carton, Dominican, harton, pelipita, purple, cachaco, popocho, pompo, maqueño, guineo and trout, being the most cultivated harton and Dominican17.
Latin America and the Caribbean have an average production in the period from 2016 to 2022 of 15465 tons, followed by South America with 9000 tons and Central America with 6,120 tons. Ecuador is the largest exporter in the world with 6,493 tons, followed by the Philippines, Costa Rica and Guatemala. Finally, Colombia is ranked number 5 with a total of 2036 tons of exports 18.
According to the Ministry of Agriculture and Rural Development, banana production in Colombia between 2016 and 2020 averaged 4171375 tons. In the period 2019 to 2020, there was a production of around 4376922 tons and 4494324 tons, making them the years with the highest production at present. The state of Caldas participated on average with 23,485.6 tons produced 19. Plantains are an important product for the economy and food security of Central and Latin America.
Plantain is an important source of starch, representing between 60% and 80% of the total fruit on a dry basis 20. This compound is a granular biopolymer that is composed of 16% amylose and 84% amylopectin 21. However, the use of native starches as a raw material in agro-industrial processes may present technological limitations in their viscosity, solubility, water retention capacity and thermal stability, among others 22. For this reason, the chemical modification of this starch is widely used to improve these properties and subsequently use it as an encapsulating material 23. Chemical modification of native starches has been extensively studied, including starches from cassava 24, corn 25, tapioca 26, and banana 27. Chemical modification occurs by esterification reactions, which involve the substitution of hydroxyl groups by ester groups. Starches can be esterified using different types of inorganic and organic acids, anhydrides and acyl chlorides 28.
One of the most studied methods to protect and preserve bioactive compounds is encapsulation by spray-drying, using modified starches as a coating material. This process has been shown to have advantages in terms of reduced processing costs, biological stability, continuous operation, controlled release, among others 29. In a study carried out by Xiao 30, the preparation and characterization of starches modified with octenyl succinic anhydride for the microencapsulation of essential oils was investigated, the results suggest that starches modified with octenyl succinic anhydride are useful as wall materials for the encapsulation of bioactive components. Microencapsulation is a technology used primarily for protecting sensitive compounds and enabling their bioavailability 31. By microencapsulating a bioactive compound, the release can be controlled over time, allowing for more sustained and efficient delivery of the compound. The choice of coating material used in microencapsulation depends on several factors, such as the characteristics of the material to be encapsulated, including its shape, solubility, stability in different solvents and against temperature, and biocompatibility and chemical compatibility with the coating material 31. According to a study by González-Aguilar et al.32, the most common materials used for the encapsulation of bioactive compounds are natural and synthetic polymers, lipids, proteins and other hydrocolloids.
The main objective of the research was to develop coffee mucilage capsules with antioxidant capacity using spray drying technology. The aim was to take advantage of coffee mucilage and provide added value, as well as to utilize an indigenous product such as banana. This work focused on the microencapsulation of bioactive compounds from coffee mucilage through the spray drying process.
Materials and methods
Raw Material
For the development of this research, coffee mucilage (Coffea) of the "Castillo" variety was used, from the National Coffee Research Center (CENICAFÉ), the mucilage was filtered in a set of 20, 30, 40, 50, 70, 100 and 140 μm mesh sieves (U.S. Standard Sieve), with the aim of removing impurities 11, then a concentration was carried out by means of a rotary evaporator (Buchi Waterbath B-480), where the ranges of the process conditions were: absolute pressure between 19.9 kPa and 25 kPa and temperatures between 60°C - 65°C, the above, with the aim of increasing the amount of solids in the sample without causing damage to the bioactive compounds of interest.
Coating Materials
Commercial maltodextrin of 20 equivalents of dextrose (ED) from the company Tencas SA and banana starch chemically modified with 2-octenyl succinic anhydride (OSA) of the variety "Dominico hartón", harvested between the 8th and 10th weeks after flowering, in a green state of ripeness, sourced from the Montelindo farm, owned by the University of Caldas, located in the municipality of Palestina, in the state of Caldas, Colombia, at an altitude of 1050 m.a.s.l., average temperature of 22.5ºC, and relative humidity of 76% were used as coating materials. The area has a rainfall of 2100 mm per year and soil of volcanic origin.
Native banana starch was obtained following the methodology proposed by Chávez-Salazar et al.33. In this protocol, the sliced banana samples are taken, then distilled water is added in a 1:1 (v/v) ratio, homogenized at 6000 rpm for 5 minutes, constantly adding distilled water, then passed through 100 µm mesh, finally centrifuged again at 6000 rpm for 4 minutes to separate the precipitate and carry out convection drying at 40°C for 48 hours.
The chemical modification of starch was carried out according to the methodology proposed by Quintero-Castaño et al.34. Method where bananas are peeled, chopped and homogenized by adding water in an industrial blender. The homogenate is filtered using a 100 µm sieve, in order to separate the starch from the rest of the components. Then, 3% (w/w) of OSA is added to the starch obtained with respect to its solids. The reaction is carried out for 4 hours at 20°C and a pH of 8.5. Said reaction is brought to a pH with a value of 7. The product is decanted, washed three times with water and finally with acetone. The starch obtained is dried at 40°C for 36 hours and subsequently stored in metallised polyethylene bags.
Characterization of coffee mucilage
Characterization methodologies are described below for fresh and filtered coffee mucilage (MF), concentrated coffee mucilage (MC), and concentrated mucilage with the addition of maltodextrin and chemically modified starch (MCMA).
Acidity
To determine acidity, the methodology established in Official Method 962.12 of AOAC International (2019) was applied. This method involves titrating the sample using a standard sodium hydroxide (NaOH) solution in the presence of the pH indicator phenolphthalein. Acidity is expressed as titratable acidity (AT), which represents the total amount of titratable acids present in the sample.
Ash
For ash determination, Official Method 942.05 of AOAC International (2019) was employed. This procedure involves incinerating the sample at high temperatures to decompose it into its inorganic mineral components, which remain as a final residue known as ash. The ash content is expressed as a percentage of the original sample weight (%A).
Antioxidant Capacity
Antioxidant capacity was determined using Official Method AOAC 2012.23, which evaluates the Oxygen Radical Absorbance Capacity (ORAC) of the sample, thus reflecting its total antioxidant activity. This procedure employs fluorescein as a fluorescent probe to measure the ability of antioxidants present in the sample to scavenge free radicals generated during the experiment.
Crude Fiber
Crude fiber determination was performed according to Official Method 962.09 of AOAC International (2019). This method involves sequential digestion of the sample with sulfuric acid and sodium hydroxide solutions, followed by a calcination process to remove residual organic matter. Crude fiber is defined as the insoluble residue that remains after this treatment.
Color Coordinates L*, b*, a*, Chroma (C) and Hue - H:
Color analysis was carried out following the methodology described by Acosta Castaño et al.35, using a Konica Minolta colorimeter, model CM-5, to measure the CIE-Lab* color space. Likewise, color change and chroma value were determined.
Minerals: Sodium, iron, calcium, potassium, and Magnesium
The minerals sodium (Na), iron (Fe), calcium (Ca), potassium (K), and magnesium (Mg) were determined following the official methodology 985.35 of AOAC International (2019). This method employs flame atomic absorption spectrometry to quantify the concentration of these elements in a solution prepared from the incinerated sample.
Moisture Content
Moisture content was determined following Official Method 930.15/90 of AOAC International (2019). This method involves drying the sample in a conventional Binder oven, model ED053-UL, at a temperature of 105°C until constant weight is reached. Subsequently, the percentage of weight loss was calculated.
pH
To determine pH, Official Method 10.041 of AOAC International (2019) was employed, using a calibrated pH meter with a combined glass electrode.
Fat Content
Fat content was determined using the Soxhlet method, following the methodology proposed by Hewavitharana et al.36. This process involves drying and grinding the sample, which is then placed in a sample holder for extraction. The solvent is heated, condenses, and drips onto the sample, extracting the fat. This extraction cycle is repeated for an extended period, around 6 hours, ensuring complete extraction. Subsequently, the solvent is evaporated, and the remaining fat is weighed to calculate the total fat content.
Protein
To determine protein content, Official Method 920.123 of AOAC International (2019) was followed. This method involves digesting the sample using concentrated sulfuric acid (H2SO4), followed by the conversion of organic nitrogen into ammonium sulfate. Subsequently, the ammonium sulfate is titrated with boric acid to determine the amount of nitrogen present. Protein content is calculated from the amount of nitrogen measured.
Stability index (MCMA)
The stability index was determined according to the methodology described by Chávez-Salazar et al.37. For this purpose, the sample dispersion (MCMA) was diluted in water in a 1:100 ratio. A UV-Vis spectrophotometer was used to measure the absorbance of the diluted sample in a wavelength range of 400 to 800 nm. The stability index (SI) was calculated as the quotient between the two absorbance readings (A800/A400).
Total sugars and carbohydrates
The determination of total carbohydrates and sugars in the samples was carried out following the Clegg-Antronhe Method 38. This method involves hydrolyzing the sample with perchloric acid to break down complex carbohydrates into simple sugars. Then, a standard glucose solution is prepared and diluted to create a series of reference solutions with known glucose concentrations. Subsequently, the Anthrone reagent is added to both the sample and the reference solutions. The Anthrone reagent reacts with glucose to produce a blue-green colored complex. The absorbance of the blue-green colored complex is measured at 630 nm using a spectrophotometer. The concentration of glucose in the sample is calculated by comparing its absorbance to the absorbance of the reference solutions using a standard curve.
Viscosity
Viscosity was determined following the methodology proposed by Chávez-Salazar et al.37 with some modifications. A Brookfield viscometer (model RVF, Stoughton, MA) equipped with an LV-01 probe and a rotational speed of 12 rpm was used to measure the viscosity of the samples. The viscosity of the samples was expressed in centipoise (cP).
Dehydration process via spray drying
This procedure was executed in a standard spray dryer "PSALAB" brand Vibrasec SAS located in the technological development center of the bioprocesses and agribusiness plant attached to the University of Caldas. The operating conditions used in the investigation were: air inlet temperature, 130 °C, air outlet temperature, 85 °C, spinning speed of the atomizing disk, 26000 rpm, maltodextrin concentration, 4.8% w/w and concentration of chemically modified banana starch, 16.8% w/w, values adapted from the methodology proposed by Quintero-Castaño et al.27. The feed to the dryer (MCMA) was homogenized by means of an IKA T18 brand ultraturrax at 10,000 rpm for 5 minutes. On the other hand, the performance of the drying process was evaluated, this was determined by comparing the total solids content of the resulting product with that of the feed introduced into the dryer.
The process is summarized in a diagram to provide a clear visual representation of the steps involved (Figure 1).
Characterization of coffee mucilage microcapsules (MP)
The coffee mucilage capsules (MP) obtained after the spray drying process were characterized using the variables and protocols described in section 2.3 (Characterization of coffee mucilage), with the exception of soluble solids, stability index, and viscosity. Other analyses conducted on the MP are described below.
Bulk density and Compacted density
The bulk density of the coffee mucilage powder (MP) samples was determined following the procedure described by Lanzerstorfer 39. The powder was allowed to fall to the bottom of a funnel to permit 120 cm³ to flow by gravity into a coaxial measuring cylinder. Subsequently, excess material was removed by passing a straight blade across the top of the measuring cylinder. To obtain the tapped density, the sample was taken from the same measuring cylinder used for determining bulk density and subjected to compression. The tapped density was calculated by measuring the mass of the compacted material and dividing it by the volume occupied by the material after compression.
Morphological analysis by Scanning Electron Microscopy (SEM)
The shape and surface morphology were determined using the methodology reported by Bhatia and Rohilla 40, examined through scanning electron microscopy (JSM-6100, Scanning microscopy, Japan). The sample was coated with gold and mounted on stubs.
Solubility
The solubility determination methodology was adapted from Olufunmi et al.41. 1 g of MP was taken and 50 mL of distilled water were added. The resulting mixture was heated to a temperature of 60°C for 30 minutes in a water bath. The mixture was then cooled to 30±2°C and centrifuged (500 rpm, 15 min). Aliquots (5 mL) of the supernatant were dried to a constant weight at 110°C. The residue obtained after drying the supernatant represented the amount of MP solubilized in water. Solubility was calculated as grams per 100 grams of MP on a dry weight basis.
Water activity
Statistical Analysis
Each of the coffee mucilage characterization variables MF, MC, MCMA and MP were measured in triplicate and analyzed by an analysis of variance (ANOVA), using the Statgraphics Centurion XVI.I. It should be noted that at the time significant differences were found (p <0.05) they were compared by a multi-rank test using the Least Significant Difference (LSD) criterion.
Results
Characterization of MF, MC and MCMA
The characterization values of the MF, MC and MCMA samples are reported in Table 1.
Table 1 Physicochemical characterization, stability index, functional, color coordinates, rheology and functional of MF, MC and MCMA
| CHARACTERISTICS | UNITS | VALUE | ||
|---|---|---|---|---|
| MF | MC | MCMA | ||
| Acidity | % | 0,27 ±0,01a | 0,18±0,01b | 0,19±0,01b |
| Antioxidant capacity | ORAC value µmoles trolox/100g | 179,20a | 902,86b | 950,68c |
| Ash | % | 0,13±0,01a | 0,88±0,01b | 0,77±0,01c |
| Calcium | mg/100g | 28,05±0,01a | 189,81±0,01b | 152,17±0,01c |
| Chroma - C | 68,63±0,05a | 62,85±0,05b | 63,28±0,02c | |
| Color coordinate a * | 10,14±0,01a | 9,24±0,02b | 11,53±0,02c | |
| Color coordinate b* | 25,92±0,05a | 18,01±0,04b | 22,90±0,04c | |
| Color coordinate L* | 43,63±0,07a | 28,95±0,01b | 36,67±0,02c | |
| Crude fiber | % | 0,01±0,01a | 0,07±0,01b | 0,78±0,01c |
| Fat content | % | 0,00±0,00a | 0,00±0,01a | 0,08±0,01c |
| Iron | mg/100g | 0,47±0,01a | 3,18±0,01b | 2,75±0,01c |
| Magnesium | mg/100g | 7,32±0,01a | 49,53±0,01b | 42,19±0,01c |
| Moisture content | % | 98,20±0,05a | 87,82±0,03b | 69,54±0,25c |
| pH | - | 4,53±0,03a | 4,50±0,01a | 4,57±0,01c |
| Potassium | mg/100g | 20,40±0,01a | 138,04±0,01b | 138,56±0,01c |
| Protein | % | 0,56±0,01a | 3,79±0,01b | 3,11±0,01c |
| Soluble solids (°Brix)) | % | 3,03±0,06a | 12,27±0,25b | 20,20±0,52c |
| Sodium | mg/100g | 22,1±0,01a | 149,54±0,01b | 120,60±0,01c |
| Stability Index | - | - | - | 0,48±0,02 |
| Sugars | % | 1,10±0,01a | 7,44±0,01b | 7,41±0,01c |
| Tone - H | 27,83±0,04a | 20,24±0,04b | 25,64±0,04c | |
| Total Carbohydrate | % | 1,11±0,01a | 7,51±0,01b | 25,58±0,01c |
| Viscosity | Cp | 151,10±3,48a | 418,17±3,06b | 985,20±4,66c |
* Average of three measurements ± standard deviation. Columns that do not share the same letter are significantly different (p <0.05). (MF) Fresh Mucilage. (MC) Concentrated Mucilage. MCMA Concentrated Mucilage with addition of Maltodextrin and chemically modified Starch.
Characterization of coffee powder mucilage (MP)
MP characterization values are reported in Table 2.
Table 2 Physicochemical characterization, color and functional coordinates, of the MP
| CHARACTERISTIC | UNITS | VALUE |
|---|---|---|
| Acidity | % | 0,48 ±0,02 |
| Antioxidant capacity | ORAC value µmol Trolox equivalents/100g | 5444,35 |
| Ash | % | 0,99±0,01 |
| Bulk density | g/mL | 0,629±0,01 |
| Calcium | mg/100g | 102,21±0,01 |
| Chroma - C | 71,47±0,05 | |
| Color coordinate a* | 5,44±0,01 | |
| Color coordinate b* | 16,24±0,01 | |
| Color coordinate L* | 76,93±0,02 | |
| Compacted Density | g/mL | 0,888±0,01 |
| Crude fiber | % | 0,85±0,01 |
| Fat content | % | 1,64±0,02 |
| Iron | mg/100g | 2,89±0,01 |
| Magnesium | mg/100g | 45,23±0,01 |
| Moisture content | % | 3,92±0,02 |
| Potassium | mg/100g | 335,23±0,01 |
| Protein | % | 2,35±0,01 |
| Solubility | % | 14,62±0,22 |
| Sodium | mg/100g | 78,04±0,01 |
| Sugars | % | 26,42±0,01 |
| Tone - H | 17,12±0,05 | |
| Total Carbohydrate | % | 91,10±0,01 |
| Water activity | - | 0,19±0,01 |
| Water Retention | % | 90,53±2,88 |
* Average of three measurements ± standard deviation.
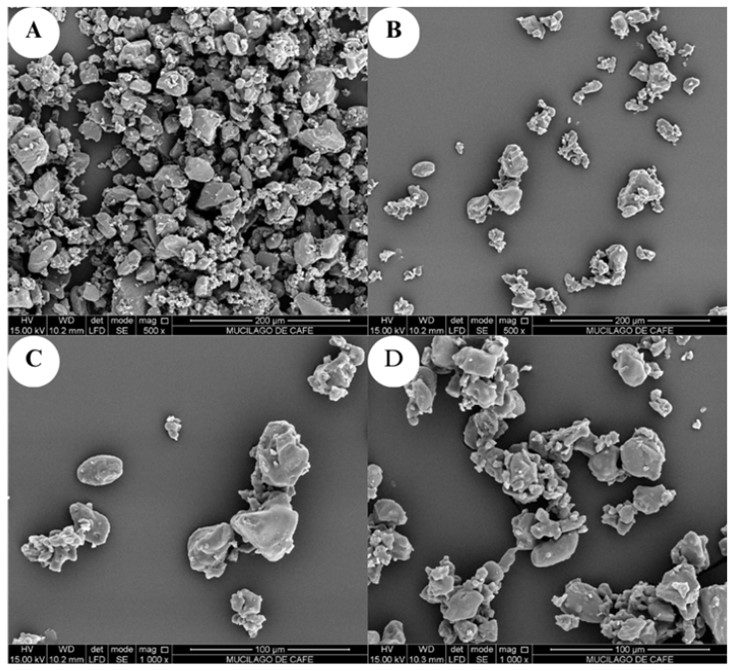
The spray-drying process is a technique widely used in encapsulation methods, a methodology by which agglomerates of certain morphologies and defined sizes are produced. In Figure 2, morphology can be observed at different zoom magnitudes (500x and 1000X) in SEM photomicrographs.
Discussion of results
Characterization of MF, MC and MCMA
In MF, the high humidity and carbohydrate values make mucilage an easily deteriorable material and, as it is a waste from the coffee industry process, it becomes a source of contamination. Table 1 shows the high moisture content (98.2% ± 0.05) and the high content of total carbohydrates (1.11% ± 0.01) with respect to their dry matter content. A similar result was reported by Hejna 42, who obtained values close to 88% in coffee mucilage, which is directly related to the result obtained in this research (151.1 Cp ± 3.48).
Currently, there are few applications that are generated from this waste, one of them has been the production of ethanol, lactic acid and hydrogen 43, as well as the production of bioalcohols 44 and the production of biogas 45. However, some entities have shown their interest in the use based on its functional characteristics as a material with a high antioxidant capacity (11,14). As a result of this research, an antioxidant capacity of 179.2 µmol trolox/100g value that resembles that reported in coffee pulp (216 µmol Trolox / g of dry matter) is reported in Table 1 42, other investigations corroborate this hypothesis, reporting the main representatives of antioxidants in coffee pulp, among which caffeine, ferulic acid, protocatechic acid, 5-caffeoylquinic acid, 3- p -coumaroylquinic acid, 3-feruloylquinic acid and tannins are mentioned, all these compounds are included in the category of total chlorogenic acids 14,46-48.
With reference to the MC, it should be noted that some substances of interest such as antioxidants are sensitive to heat, which is why the MF was subjected to a concentration by vacuum rotary evaporation which decreased its moisture content by 10.38%. The antioxidant capacity of the MC finally reached a value of 902.86 µmol trolox/100g significantly different from the MF value, increasing by 723.66 µmol trolox/100g, a result that infers that the temperature and pressure conditions used in the concentration process were adequate while preserving the functional characteristics of the substances present in the MF. Similarly, the MCMA recorded a lower moisture content and statistically different from the MF and MC, this result is due to the contribution of solids from maltodextrin and modified banana starch.
In the case of viscosity, there were statistically significant differences between the MF, MC and MCMA samples, increasing in the latter to 985.2 Cp, this property was directly affected by the increase in solids, a value that remained within the working limit of the pump that feeds the atomization unit in the spray dryer. Likewise, the stability index of the MCMA was 0.48±0.02 and although this value is below those reported by Carlos et al.49 and Chávez-Salazar et al.37, the dispersion did not show characteristics of instability such as phase separation or sedimentation. Mirhosseini et al.50, meanwhile, clarify that the stability index is based on the scattering properties of light and that this characteristic is related to the average particle size.
Specifically, with regard to the antioxidant capacity of MCMA, an increase of 47.82 µmol trollox/100g was found with respect to MC, and this is due to the contribution of some compounds with antioxidant capacity present in the modified banana starch. Borges et al.51, mention that banana and banana fruits (Musa spp.) are considered a good source of antioxidants. Similarly, Sarawong et al.52, report that currently flour from green bananas is of interest for its nutritional value, especially its high amount of resistant starch, dietary fiber and bioactive compounds such as phenolic acids.
In Table 1, the significant changes in the color coordinates of each of the MF, MC and MCMA mucilage samples can be observed, variations that place this variable in a shade between red and yellow being closer to the red color, with a chroma or dark saturation that matches the visual perception of the brown or brown samples. In terms of luminosity, it is the MF that has a higher value, which makes it clearer or more translucent compared to the MC and the MCMA.
Characterization of coffee powder mucilage (MP)
The characterization of the MP sample obtained by the spray-drying process is shown in Table 2. In this process, a moisture content of 3.92%±0.02 was reached, implying a significant change in the composition of the sample with respect to the MCMA, as can be seen in the antioxidant capacity, a characteristic that reported a statistical significance of 5444.35 µmol trollox/100g, increasing by 4493.67 µmol trollox/100g. Therefore, it can be concluded that maltodextrin and chemically modified banana starch in conjunction with the spray drying process conditions, provided protection to the components of interest with antioxidant capacity initially contained in the MF. Some authors have reported studies where native or modified starches, maltodextrins, gums and other substances have been used as wall materials that provide protection in the spray-drying process. The findings of this study unveil notable distinctions between the properties of MP and the microencapsulated L-ascorbic acid in spherical aggregates of taro starch by spray drying reported by Hoyos-Leyva et al.53. Firstly, the moisture content of MP is considerably lower (3.92 ± 0.02%) compared to taro starch (5.9 ± 0.15%), indicating enhanced product stability and a reduced risk of microbial growth or degradation in MP. Similarly, the water activity in MP is significantly lower (0.19 ± 0.01) than in taro starch (0.35 ± 0.01), further supporting the superior microbiological stability of the material. In terms of density, MP exhibits a much lower density (0.629 ± 0.01 g/mL) compared to taro starch (1.1 g/mL), suggesting a lighter material potentially more suitable for certain applications. On the other hand, the solubility of MP is considerably lower (14.62 ± 0.22%) in contrast to taro starch (70.09-91.26%), attributed to the specific composition of the encapsulated material, in this case, coffee mucilage. Regarding the morphology of the microcapsules, the microcapsules exhibit a distinct structure. These findings highlight the significant differences between MP and the results obtained by Hoyos Leyva et al.53, suggesting distinct applications and considerations for each in terms of stability, density, solubility and morphology.
In other study, made by Karrar et al.54 investigated the encapsulation of gurum seed oil using a combination of maltodextrin, gum arabic, and whey protein isolate via spray drying. The results demonstrated successful oil encapsulation within microcapsules, and morphological analysis revealed spherical microcapsules with no apparent surface cracks. Our study yielded similar results in terms of moisture content (3.67%). Another important property is the solubility of coffee mucilage powder. Compared to Karrar's study (86-93.84%), our solubility was significantly lower (14.62 ± 0.22%). This difference can be attributed to the chemical composition of coffee mucilage, which contains pectic compounds like protopectins, pectic acids, and pectins, known for their strong intermolecular bonds that limit solubility 55. While maltodextrin is water-soluble, its ability to enhance mucilage solubility may be restricted by the mucilage's pectic nature and intermolecular linkages. Despite the chemical modification of starch with OSA, which is supposed to increase solubility 56, it appears insufficient to overcome the mucilage's inherent low solubility. Furthermore, process conditions, such as drying speed, can influence the powder's final solubility. As observed in SEM images, the particles exhibited minimal porosity, which, coupled with the mucilage's pectic composition, could hinder MP dissolution. The powder's physical properties, such as apparent density, can also affect solubility. A powder with higher apparent density may impede water penetration, consequently affecting solubility 57. Therefore, solubility is an important factor in designing encapsulation systems for specific applications.
In this research, the moisture content of MP was determined, being a determining property for its stability during storage. In this study, a moisture value of 3.92±0.02% was obtained, which aligns with the findings of Mahdi et al.23, who reported similar values for microcapsules. Water activity (Aw) is another crucial factor influencing the microbiological stability of microcapsules. An Aw value below 0.6, offers a double benefit: it significantly hinders microbial growth (moulds, yeasts, and bacteria) and promotes greater chemical and biochemical stability. This lower Aw helps prevent some reactions that could lead to undesirable changes in the product. Mahdi et al. observed an Aw range of 0.23 to 0.33 in their microcapsules, while in this study, the water activity was 0.19±0.01. These low Aw values and moisture content restrict microbial growth and contribute to a longer product shelf life. Regarding density, a value of 0.629±0.01 g/mL was obtained in this research, falling within the range reported by Mahdi et al. for the combination of gum arabic, maltodextrina and modified starch (0.60 g/mL). A higher apparent density indicates less air in the powder, which can be desirable for certain applications, such as protection against lipid oxidation during storage. On the other hand, solubility in our research was lower compared to that reported by Mahdi et al., as it was 14.62±0.22 in our study, while Mahdi's study yielded values around 70.09-91.26%. This difference could be related to the specific composition of the encapsulated material, in this case, coffee mucilage. Scanning electron microscopy (SEM) allowed for the observation of microcapsule morphology. In contrast to the findings of Mahdi et al., where microcapsules exhibited a spherical shape, our study revealed microcapsules with a distinct morphology characterized by particulate material, roughness, and the presence of agglomerates.
In this research, other characteristics of the MP were analyzed, which are listed in table 2. The reported water activity (Aw) of 0.19 ±0.01 is consistent with the results of some investigations. Martins et al.58 evaluated this characteristic in Lactococcus Lactis and obtained a value of 0.198. Other authors report slightly higher Aw values (0.28) in spray-dried melon juice 59) and 0.24-0.27 for amylose-lipid complexes in powders similarly obtained by the spray-drying method 60.
The results of solubility (14.62%±0.22) and water retention (90.53±2.88%) in the MP, can be related to the ratio used of maltodextrin and chemically modified banana starch in the spray drying (1/3.5) and to the percentage of modified starch used (16.8%), values that help to favor the water retention characteristic. This type of behavior influences the possible applications in various food matrices. For their part, solubility and water retention are parameters that determine the reconstitution quality of the powder. Etzbach et al.61, evaluated the effects of the carrier agents on the properties of the powder, stability of the carotenoids and the encapsulation efficiency of goldenberry powder (Physalis peruviana L.) produced by co-current spray drying and found that powders produced with maltodextrin or modified starch revealed high solubilities in cold water (> 90%), they also attribute this effect to the hydrophilic character of maltodextrin and the amphiphilic character of the modified starch that could favor simultaneous interaction with polar and non-polar constituents; however, the methodology used by these authors favored a high solubility in cold water due to the long exposure times with agitation (30 minutes).
On the other hand, density is an inherent characteristic of powder products and is often related to their storage behaviour, thereby affecting the quality of such powders. Table 2 shows the values of bulk density (0.629±0.01 g/mL) and compacted density (0.888±0.01 g/mL), values that are within the ranges reported for flours and starches by Lanzerstorfer 39, who evaluated the bulk density of compressible food powders under storage conditions with the help of some mathematical equations that helped predict the behavior in said storage, based on the height and angle of wall friction inside the silo.
The MP microstructure (Figure 1), show the particles have amorphous forms with an average measurement of 29.16±2.12 µm. Some of the particles have oval shapes and this may be due to the presence of modified banana starch, a material that has this same tendency 33. On the other hand, agglomerates with slightly rough surface can be observed, possibly due to damages caused during the spray drying process, such as, for example, slow or fast crusts formed around the chamber due to the effect of the speed of the atomization disk, feed flow to the atomization unit, among others. However, the encapsulation and coating effect of the compounds of interest with antioxidant capacity power was achieved, as shown in Table 2 (5444.35 µmol trolox/100g), it is possible that the agglomerates formed and shown in Figure 1 fulfill that function. Some authors report agglomerates of spherical shape and with greater encapsulating power in some compounds of interest, attributing these characteristics to the size and shape of the particles of the starches used in the spray-drying process, as is the case of Hoyos-Leyva 53 who evaluated the physical and chemical stability of l-ascorbic acid microencapsulated with taro starch by spray-drying and Yun et al.60 who evaluated the physical, microstructural and digestive properties of amylose-lipid complexes using rice flour as a starch source in different drying methods in which spray-drying was included.
Conclusions
A significant increase in the antioxidant capacity of coffee mucilage powder (MP) was achieved, from 950 to 5400 mmoles of Trolox/100 g. This increase is attributed to the protection and encapsulation of the antioxidant compounds present in the mucilage, an effect enhanced by maltodextrin and OSA-modified starch.
The spray drying process with maltodextrin and OSA starch produced coffee mucilage agglomerates that effectively encapsulated the antioxidant compounds, protecting them from degradation during drying. Although the expected capsule shape (spheres) was not observed, the morphology of the agglomerates did not negatively affect the powder's functionality.
Encapsulation provided coffee mucilage powder with greater stability and resistance to the environment, allowing for its long-term preservation and use. This powder has high potential as a functional ingredient in the food industry due to its antioxidant capacity and encapsulating properties.
Application studies are recommended to evaluate the effectiveness of coffee mucilage powder in various food matrices. Additionally, further research is needed to fully understand the mechanisms by which maltodextrin and OSA starch enhance the antioxidant activity of coffee mucilage.
This research contributes to the strengthening of the coffee and banana production chains in Colombia by valuing coffee mucilage as a source of natural antioxidants. This opens up new opportunities for product diversification and value addition. Although more research is needed in this area, so far, this type of research contributes to the strengthening of the coffee and banana production chains in Colombia.