Introduction
Dioscorea is one of the six genera of the Diosco-reaceae family, which is comprised of more than 650 species distributed in tropical areas with high rainfall (Thurston, 1998). The Dioscorea sp. yam is a very important tuber for the small-scale producers of the Caribbean Region in Colombia. Many of this region's farming families depend on this crop (Reina, 2012), as well as the markets that are part of the free trade agreements (FTA). Therefore, developing research aimed at understanding its features and finding solutions to the problems presented by the crop is of vital importance.
In the Sucre Department, the most cultivated species are the "white yam" (Dioscorea rotundata) and "purple yam" (Dioscorea alata) with 75% of the total amount cultivated (Reina, 2012). In this area of the country, yam is produced from vegetable form through division of the tubers into portions, causing the transfer of diseases from one cycle to another and from one locality to another, in some cases (Rodríguez, et al. 2008). Therefore, the initiative arose to implement new methodologies for the conservation of this species, such as in vitro culture. Through this methodology, the Universidad de Sucre established a germoplasm bank in which the previously mentioned species are protected.
The conservation of this crop in a germoplasm bank constitutes a solution to the problems presented by field cultivation. There are reports about the micropropagation of some edible yams, such as D. rotun-data cv. "white yam" (Acosta and Beltrán, 2001); D. alata cv "Pico de Botella" (Rodríguez and Beltrán, 2001); and somatic embryogenesis in D. alata cv "Diamante 22" (Espitia and Quintero, 1999) (cited in Salazar, 2002). However, the in vitro cultivation of Dioscorea spp. presents disadvantages, such as short periods between subcultures (3 to 4 months), which as a consequence, entails increases in costs and the use of labor, affecting the genetic stability of the accessions and compromising their asepsis (Acedo and Arradoza, 2006).
Therefore, it was necessary to apply a new conservation method that would allow the subculture periods to be extended and thus prevent the loss of material and increases in costs and labor. This method is called conservation by minimum growth, which aims to directly restrict the growth and development of a plant through controlled osmotic stress generated by some components of the culture medium, such as sugars and alcohol sugars in combination with controlled environmental parameters (Neiva and Jiménez, 2010). There are conservation reports on Dioscorea spp., such as "Dioscorea alata L, "Caraqueño" clone (Borges et al. 2009). In this study, they managed to conserve and regenerate in vitro plants from uninodal segments of the D. alata L "Caraqueño" clone in 9 to 12 months with different concentrations of mannitol, sucrose and BAP. Studies have also been carried out on other species such as "Dioscorea alata, D. rotundata, D. bulbifera and D. trífida" (Carmona, et al. 2013), which managed to conserve four in vitro yam species using a combination of sucrose, manni-tol and sorbitol for a period of more than six months. These methods have been successful, however, in this last research, the species responded differently to the different treatments, so each species had a medium in which it was maintained in the adequate conservation conditions during the research.
For the reasons expressed above, the objective of this work was to establish optimum culture media for the in vitro conservation by minimum growth of different accessions belonging to the yam germoplasm bank of the Universidad de Sucre. This was for a period of 8 months and based on the modification of the culture medium with different levels of mannitol and sorbitol, individually and combined.
Materials and Methods
Geographical Location
This research was conducted in the Laboratory of Biotechnology and Cultivation of Plant Tissue of the Universidad de Sucre, Puerta Roja Campus. This university is located in the city of Sincelejo with the geographical position in Colombia of 9° 18' north latitude and 75° 23' west longitude of the prime meridian (Ortega et al. 2011).
General Procedures and Techniques
Obtaining Plant Material
The micropropagation media were prepared for the establishment of mother plants. These consisted of M&S salts (Murashige and Skoog, 1962), sucrose 30 g/L, activated carbon 2 g/L, ANA 0.5 mg/L, BAP 4 mg/L, thiamine 1 mg/L, myoinositol 0.1 g/L, and agar 8 g/L for Dioscorea alata. For Dioscorea ro-tundata, media were prepared with M&S salts (Murashige and Skoog, 1962), sucrose 30 g/L, activated carbon 2 g/L, ANA 0.1 mg/L, BAP 0.3 mg/L, thiamine 1 mg/L, myoinositol 0.1 g/L, and agar 3.25 g/L.
The pH of the culture medium was adjusted to 5.8 and distributed in flasks of 182 cm3, at a ratio of 20 ml per flask. Finally, it was sterilized for 20 minutes at 15 psi and 121 °C. The media were observed for seven days before their use to rule out preliminary contamination.
Three segments were planted per flask with nodes and leaves of the donor plants from the germoplasm bank of the Plant Biotechnology Laboratory of the Universidad de Sucre. The activities to prepare equipment, sterilize materials and culture media were implemented under aseptic conditions in a horizontal laminar flow cabinet (Astrocel®) in controlled temperature and lighting conditions.
Planting of Explants and Cultivation Conditions
Single-node (without leaves and roots), approximately 1 cm-long segments were used, which were obtained from the micropropagated mother plants at three months from their establishment. The segments were planted, according to the polarity of the plant at the ratio of one explant per flask under a horizontal laminar flow cabinet in aseptic conditions. Finally, they were placed in an incubation room at a temperature of 28 ± 5 °C, relative humidity of 65% and a photoperiod of 12 hours of light with a light intensity of 50 μmol m-2s-1.
In Vitro Conservation
Culture Media
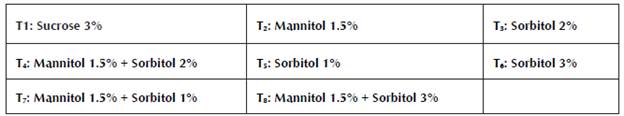
These consisted of M&S salts (Murashige and Skoog, 1962), activated carbon 1 g/L, ANA 0.5 mg/L, BAP 4 mg/L, thiamine 1 mg/L, myoinositol 0.1 g/L, and agar 8 g/L for Dioscorea alata. For Dioscorea rotundata, media were prepared with M&S salts (Murashige and Skoog, 1962), activated carbon 1 g/L, ANA 0.1 mg/L, BAP 0.3 mg/L, thiamine 1.0 mg/L, myoinositol 0.1 g/L, and agar 8 g/L. The medium was modified with different concentrations of sucrose (%), mannitol (%) and sorbitol (%), individually and combined. They consisted of the following treatments: T1 (3:0:0); T2 (0:1, 5:0); T3 (0:0:2); T4 (0:1, 5:2); T5 (0:0:1); T6 (0:0:3); T7 (0:1, 5:1); and T8 (0:1, 5:3). The pH of the culture medium was adjusted to 5.8, then the agar was dissolved for ten minutes. on a heating plate and distributed in flasks of 182 cm3 at a ratio of 20 ml per flask. Finally, it was sterilized in an autoclave (Sterilof®) for 20 minutes at 15 psi and a temperature of 121 °C . The media were maintained for seven days (maximum) before being used to rule out any contamination of them. The media of the different treatments were planted in aseptic conditions in a horizontal laminar flow cabinet (Astrocel®).
Evaluation of Conservation
The following variables were measured every 30 days over a period of eight months.
- Length of stem (measured in centimeters with a millimeter ruler from the base of the explant to the last node).
- Length of longest root (cm).
- Number of nodes per explant.
- Number of roots.
- Percentage of expanded green leaves (number of expanded leaves / total number of leaves).
- Percentage of dead leaves (number of dead leaves / total number of leaves).
- Callus percentage (number of in vitro plants that generated calluses / total number of plants).
- Oxidation percentage (number of in vitro plants with darkening in the tissue / total number of plants). It was assessed by visual observation.
- Survival percentage (number of live in vitro plants / total number of plants), Estrada et al. (2009).
Design and Statistical Analysis
This research was conducted under a completely randomized design (CRD) comprised of eight treatments with three repetitions and eight replicas. Normality tests (Shapiro-Wilk) and homogeneity of variance (Bartlett) tests were applied to the data obtained, after which, the normally and homogeneously distributed variables were subjected to an analysis of variance (ANOVA), followed by Tukey's test of multiple comparison of means (α: 0.05), while the non-parametric Kruskal-Wallis test was applied to the other variables. All of the statistical analyses were processed in the R program for Windows (De Mendiburu, 2012).
Results and Discussion
Experiment 1: Effect of Mannitol and Sorbitol on the in Vitro Conservation of Dioscorea Alata
The results obtained in this research confirm that the use of the different osmotic agents affects in vitro survival of the D. alata species. In this sense, the individual use of mannitol in the culture medium of this species is not advisable, because it causes the death of all the explants. This is possibly due to low availability of nutrients and carbon because of the limited absorption of water by the plant caused by the reduction of the culture medium's water potential, as a result of the addition of said osmoregulator. This phenomenon has been observed by Cárdenas and Villegas (2002), who found that the use of mannitol as an osmoregulator generates more negative osmotic potentials than sorbitol (Table 1).
Table 1 Percentages of survival, expanded leaves, dead leaves, calluses and oxidation in Dioscorea alata at eight months of in vitro conservation.

Subtitle: Percentages with different letters by columns significantly differ for p < 0.05, where (*) is a desirable attribute for in vitro conservation and (-) is an absent value due to death of the explants.
On the other hand, the use of sorbitol (3%) or a mannitol-sorbitol combination (1.5 - 2%), allows greater rates of survival to be achieved and therefore, it is possible to ensure the availability of this crop for those who need it. The explanation for this phenomenon is associated with sorbitol's capacity to generate less severe osmotic stress than other osmoregulators and its use as a source of energy for the explants (Table 1).
When measuring the percentage of the expanded leaves, it was found that this variable responds differently to the treatments used with T3, T7 and T8, obtaining the lowest values. Differences were also observed in leaf size. The leaves generated by culture media with sucrose and sorbitol were larger than those obtained using mannitol. This was probably due to the lack of nutrients for the development of these structures (Table 1).
Regarding leaf senescence, use of the mannitol-sorbitol combination (1.5 - 2%) is the most appropriate, because the appearance of dead leaf tissue is almost zero, possibly due to the limited development of explants growing in these conditions. Additionally, the stimulating effect of sorbitol on necrosis of the leaf tissue must be taken into account, especially when it is used individually. Similar results were obtained by Carmona et al. (2013), who found that use of the mannitol-sorbitol combination prevents leaf senescence in the Dioscorea alata in vitro plants
On the other hand, in this research, it was found that the use of different osmoregulators does not promote oxidation, nor the appearance of unorganized cell tissue (callus) in the explant (Table 1).
As shown in Table 2, the length of the stem varies depending on the source of carbon used with the T7 and T8 treatments presenting the least development. Therefore, the effect of the interaction between the mannitol and sample is shown, which causes a considerable reduction in the growth of the explant leading to "dwarf" plants, characterized by small stems and leaves, as well as nodes very close to each other. This phenomenon is the result of the interaction of the plant genotype with an inert sugar such as mannitol together with a moderately metabolizable sugar such as sorbitol. These results do not coincide with those obtained by Borges et al. (2009), who found that use of mannitol does not significantly affect the length of the stem during in vitro conservation of the Dioscorea alata "Caraqueño" clone species.
Table 2 Stem length, number of roots, length of root and number of nodes in Dioscorea alata at eight months of in vitro conservation.

Subtitle: Percentages with different letters by columns significantly differ for p < 0.05, where (*) is a desirable attribute for in vitro conservation.
Additionally, the number of roots in this species varies depending on the osmoregulator used with the use of low concentrations of sorbitol causing the lower development of this variable, which is advisable for in vitro conservation, since it permits the restriction of nutrient absorption by the explant. However, when using sorbitol concentrations over 1%, individually or combined, a larger number of roots is produced.
Therefore, although the use of sorbitol together with mannitol generates a greater number of roots, these are characterized by being small, possibly because the plant needs to increase the amount of structures to absorb nutrients from the medium to supply its metabolic needs. Therefore, the explants in these treatments "prefer" to increase the number of roots even if their size is greatly reduced.
On the other hand, the results obtained show that use of different sources of carbon does not affect the formation of nodes in the Dioscorea alata species at eight months of in vitro conservation. These results coincide with those obtained by Borges et al. (2009).
Based on the above, it is possible to confirm that the Dioscorea alata species responds most adequately to the T4 treatment. In other words, the combined use of mannitol-sorbitol in the culture medium permits the in vitro conservation of this species for eight months, because this treatment has the highest rate of survival and the lowest percentage of leaf senescence, as well as presenting restricted development in the rest of its variables (Figure 1).
Experiment 2: Effect of Mannitol and Sorbitol on the in Vitro Conservation of Dioscorea Rotundata
The results obtained in this test show that the rate of survival in the Dioscorea rotundata species is affected by the use of different osmoregulators, with the T1, T3, T4 and T7 treatments presenting the greatest values and therefore, considered appropriate for the in vitro conservation of this species. Furthermore, the individual use of mannitol in the culture medium is inadequate, because the rate of survival in these conditions is too low. These results do not coincide with those reported by Carmona et al. (2013), who found that the rate of survival in the Dioscorea rotundata species is not affected by the use of different osmotic regulators, especially when using mannitol. A possible explanation for this phenomenon may be related to the genotype of the plant material used, since they belong to different cultivars.
Additionally, the results obtained show that the percentage of expanded and dead leaves also varied with respect to the type of carbon source used. Therefore, the use of the mannitol-sorbitol combination generates reduced values of this variable, thus showing a delay in the growth and development of the explants.
Likewise, the evaluation of leave senescence in the plants indicated that the mannitol-sorbitol combinations are adequate for in vitro conservation, provided that high concentrations of sorbitol are not used (above 2%), because in both tests, the presence of sorbitol is usually associated with high percentages of leaf senescence. Similar results were obtained by Carmona, et al. (2013).
With regard to the oxidation and callus percentages, similar results were obtained to those presented by Dioscorea alata. In other words, there was not a significant effect on the appearance of these characteristics when using different sources of carbon.
The results obtained show that reduction in the growth of the stem may be achieved through the use of the manitol-sorbitol combination or the application of sorbitol in concentrations over 1%. Likewise, it is possible to achieve a considerable reduction in the formation and elongation of roots through the use of said combination. Therefore, these conditions are advisable for the in vitro conservation of this species, that is the T4 and T7 treatments. Additionally, the results indicate that there is not a significant effect on the formation of nodes on in vitro plants when using different osmo-regulators in the culture medium.
Finally, taking into account the previously mentioned proposals, it is possible to confirm that the T7 and T4 treatments are the most adequate for the in vitro conservation of the Dioscorea rotundata species, as they allow their growth and development to be significantly reduced in terms of the variables of stem and root length, as well as the number of roots, maintaining high rates of survival and a minimum percentage of leaf senescence (Figure 2).
Table 3 Percentages of survival, expanded leaves, dead leaves, calluses and oxidation in Dioscorea rotundata at eight months of in vitro conservation.

Subtitle: Percentages with different letters by columns significantly differ for p < 0.05, where (*) is a desirable attribute for in vitroconservation.
Table 4 Stem length, number of roots, root length and number of nodes in Dioscorea rotundata at eight months of in vitro conservation.

Subtitle: Percentages with different letters by columns significantly differ for p < 0.05, where (*) is a desirable attribute for in vitro conservation.
Conclusion
Finally, we can conclude that the medium comprised of salts (M&S) + mannitol 15 g/L + sorbitol 20 g/L + activated carbon 2 g/L + thiamine 1 mg/L + myoinositol 0.1 g/L + agar 8 g/L allows the effective conservation of in vitro plants from single-node segments of Dioscorea alata supplemented with 4 mg/L of BAP and 0.5 mg/L of ANA, and Dioscorea rotundata supplemented with 0.3 mg/L of BAP and 0.1 mg/L of ANA, for eight months with high survival percentages and low percentages of leaf senescence, showing restricted development of in vitro plants.











 text in
text in