Introduction
The upflow anaerobic sludge blanket (UASB) is the most used reactor in high rates of anaerobic treatment processing. The UASB is a reactor capable of retaining a high concentration of microorganisms and a waste 28, 2015 stabilization rate, and could be an option to generate biological hydrogen (H2). This reactor has positive characteristics, such as permitting a high rate of organic load, short hydraulic retention time (HRT) and a low energy demand (Metcalf and Eddy, 2003).
There is a great variety of specific bioreactor designs applied to the anaerobic treatment of waste water. These consist of complete-mix, immobilized or fixed-bed, fluidized-bed or partitioning reactors. In the application of high-speed systems, such as the upflow anaerobic sludge blanket (UASB) or expanded granular sludge bed (EGSB), the hydraulic retention times are disassociated from the solid retention times, facilitating the elimination of dyes from the waste water (Van Lier, 2001). The upflow anaerobic sludge blanket (UASB) was designed in Holland by Gatze Lettinga and collaborators at Wageningen University, conducting research from 1971 in search of an anaerobic treatment with a single passage for industrial waste water (Lettinga et al. 1980).
The most significant aspect of these anaerobic reactors is the formation of granular sludge, and they essentially consist of a dense mixture of bacteria that carry out the oxidation of organic matter into methane. The formation of grains depends on the characteristics of the residual current, the substrate load, and the operating details of the reactor, as well as the speed of the up-flow, which is from 1 to 2 m/h (it is recommended for the daily average not to exceed 1 m/h). Expanded bed UASB have been studied, managing speeds from 6 to 12 m/h. The hydraulic retention time may be one day or less and the contaminating load it can manage is from 0.5 to 40 kg/m3 (Droste, 1997). A feasible option to solve the problem of the textile effluents is the application of biological processes that degrade the dyes. The water contaminated with these compounds may be treated through similar bioreactors to the conventionally used ones, just improving the process with the stimulation of microorganisms adapted to the degradation of the dye so that it is efficiently reduced.
Razo-Flores et al. (1997) demonstrated that some azo dyes can be a source of carbon, nitrogen and energy for anaerobic microorganisms, and therefore, they can be degraded in biological reactors. They specifically used an upflow anaerobic sludge blanket (UASB). Activated carbon has been used by several researchers as biofilm support to remove contaminants in an aqueous solution using either fluidized or fixed-bed reactors.
In the case of azo dyes, the first step of the degradation is to rupture the azo bond. This step is carried out by a variety of cytoplasmic enzymes with low specificity for the substratum called "azoreductase". In anoxic conditions, these enzymes facilitate the transfer of electrons through flavins soluble in azo dye, which is reduced (McMullan, 2001). Van Der Zee et al. (2003) demonstrated that the activated carbon works as a re-dox mediator in the degradation of azo dyes, accelerating their reduction. The carbon acts in such a way that it accepts electrons from the microorganisms and transfers them to the dye molecule through the groups in its surface.
As previously mentioned, anaerobic reactors are suitable for removing azo dyes, but the degradation is very slow. Consequently, it is necessary to seek the adequate conditions to accelerate the reduction process in order to have an efficient and economical process.
Therefore, the aim of this work was to evaluate the efficiency of anaerobic biodegradation of brl direct blue dye in an upflow anaerobic sludge blanket (UASB) with activated carbon.
Materials and Methods
Collection of Samples
Wastewater samples were taken from the industrial effluents of "Rio Seco Industrial Park (PIRS, for the Spanish original)" located northeast of the center of Arequipa, Peru (71°35'59" West; 16°21'22" South; 2,250 m.a.s.l). The wastewater characteristics are shown in Table 1.
The 15 liters of sludge from the waste water of the PIRS were used as an inoculum for the reactor. Then, it was fed with the brl direct blue dye prepared in the Cellular Biology Laboratory of the Academic Department of Universidad Nacional de San Agustín (Arequipa, Peru).
Assembly of the Upflow Anaerobic Sludge Blanket (UASB) with Activated Carbon
An upflow anaerobic sludge blanket (UASB) reactor was put in operation with a capacity of 14.4 liters of manufactured acrylic with a fixed bed of activated carbon of approximately 40% of the work volume. The reactors were designed according to the method based on organic load and the flow velocity criteria (González & Escamilla, 2006).
The reactor has a tubular diameter of 15 cm with a minimum flow of 10 ml/min, a load of 6 kg COD/m3/ day and a hydraulic retention time (HRT) of one day (Figure 1).
Preparation of the Inoculum and Maintenance Conditions
Six liters of waste water were taken from PIRS industrial effluents with domestic, tannery and industrial company discharges, presenting a consortium of anaerobic microorganisms. This volume of waste water was left for two days in a hermetic, conditioned recipient that did not allow the entry of air in order to permit the development of the bacterial consortium. On the third day, 500 ml of anaerobic sludge from an activated sludge plant for the treatment of industrial effluents were added. On the eighth day, 50 g of yeast extract and 30 g of dextrose were added. From this point, 5 g/l of yeast extract and 3 g/l of dextrose were added as a source of carbon and hydrogen on a weekly basis to facilitate the formation of biomass, as well as 2 g of brl direct blue dye (azo dye), as it was the test molecule (González & Escamilla, 2006).
During the preparation of the inoculum through continuous flow, the pH, COD, total dissolved solids (TDS), total suspended solids (TSS) and volatile suspended solids (VSS) were monitored every four days. In this stage, the physicochemical parameters were measured, mainly for the conditioning of the UASB reactor, taking regular measurements up to 32 days (Figure 2).
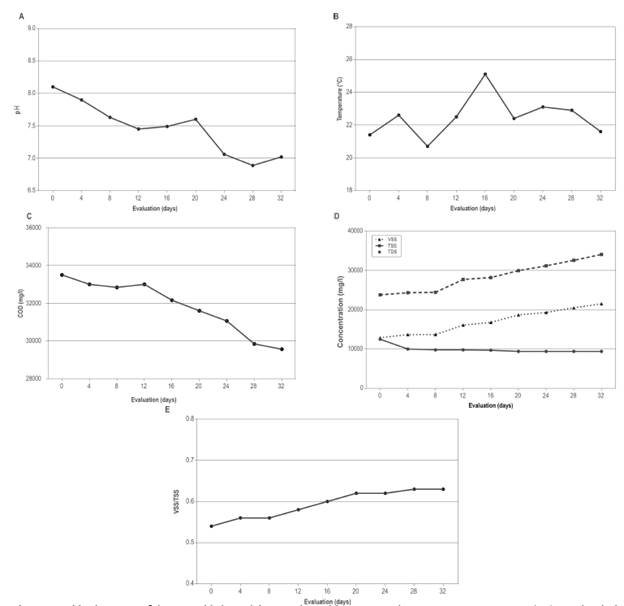
Figure 2 Cultivation stage of the anaerobic bacterial consortium with respect to time. A. pH; B. Temperature (°C); C. Chemical oxygen demand (mg/l); D. Total suspended solids (mg/l), volatile suspended solids (mg/l) and total dissolved solids (mg/l); and E. Volatile suspended solids (mg/l) / Total suspended solids (mg/l).
Operation of the UASB Reactor
The activated carbon facilitated the removal of the dye because of the active sites and because of the surface it provides for the growth of the bacterial consortium. The reactor was filled up about 40% so that the sludge bed consisted of a fixed bed of activated carbon and biomass of the bacterial consortium, making it possible to feed the reactor with a peristaltic pump.
To inoculate the reactor, it was fed for 14 days in recirculation with 20 liters of solution with 10% of the adapted bacterial consortium. This stage was called start-up stage (Figure 3). Then, it was continuously fed for 28 days using a solution with brl direct blue dye (C3oHi6Cl2N4Na2O8S2) at 70 mg/l, enriched with dextrose and yeast extract. Both improved the removal by being a source of carbon and nitrogen for the bacterial consortium, as well as providing the removal reaction. This stage was called operating stage (Figure 4) (González & Escamilla, 2006). Both stages were repeated three times.

Figure 3 Physicochemical parameters in the start-up stage of the UASB reactor for the biodegradation of the brl direct blue textile dye with respect to time. A. Temperature; B. Chemical oxygen demand (COD); C. pH; D. Total dissolved solids (TDS); E. Suspended solids (SS); and F. Turbidity expressed in FAU (Formazin Attenuation Units).
The operating stage of the UASB reactor measured the pH, temperature, chemical oxygen demand, residual dye concentration, total dissolved solids, suspended solids (SS) and turbidity in the two components of the system: UASB reactor and treated effluent. These parameters were assessed every seven days. The removal of brl direct blue dye was assessed in each component of the UASB reactor by measuring the residual concentration of the dye through spectrophotometry at a wavelength of 520 nm (Figure 5).

Figure 4 Physicochemical parameters in the operating stage of the UASB reactor for the biodegradation of the brl direct blue textile dye with respect to time. A. pH; B. Temperature; C. Chemical oxygen demand (COD); D. Removal of chemical oxygen demand (COD); E. Total suspended solids (TSS); F. Suspended solids (SS); and G. Turbidity expressed in FAU (Formazin Attenuation Units).
Adsorption of Brl Direct Blue Dye in Activated Carbon
Parallel to this, an adsorption capacity of 125 mg/l of granular activated carbon over the test dye was determined. To determine the adsorption capacity of solitary activated carbon, columns of acrylic were conditioned with two liters of brl direct blue dye at 70 mg/l and with a 40% volume of granular activated carbon. The residual concentration of the dye was monitored every seven days up to 28 days.
Analysis of the Parameters
The parameters measured in situ were the pH (Fisher Portable pH Meter), temperature (Checktemp Digital Thermometer), and total dissolved solids (Myron L Company Equipment). The parameters assessed in the laboratory were: suspended solids (photometric method with HACH DR/890 Colorimeter), volatile suspended solids (gravimetric method with vacuum pump, Jb Industries brand, origin USA) (APHA, 1992), dye concentration (absorptiometric method, spectrophotometer model S - 22 of the Kert-Lab brand), and chemical oxygen demand (digestion method with potassium di- chromate, HACH DR/890 Colorimeter) (APHA, 1998).
Statistical Analysis
For the evaluation of the physicochemical parameters in the upflow anaerobic sludge blanket (UASB) reactor, trend curves were used to observe their evolution over time for each stage of the system. For the removal of the brl direct blue dye in the reactor, the one-way ANOVA statistical analysis test was used with Tukey's range test to establish the resulting differences for 95% and 99% confidence levels in the residual concentration of the brl direct blue dye in the operating stage. The SPSS version 20 and GraphPad Prism 6 statistical packages were used to apply the statistical techniques.
Results
Cultivation Stage
An important decrease is shown in the pH values up to 32 days with a value of 7.02, while the temperature values reached a maximum of 25.1 °C after 16 days, and a temperature of 21.6 °C after 32 days. There was a decrease in COD from 33,500 to 29,560 mg/l, an increase in the TSS from 23,820 to 34,079 mg/l, and an increase in VSS from 12,894 to 21,546 mg/l. The TDS decreased from 12,500 to 9,400 mg/l, and the VSS/TSS proportion shows an increase from 0.54 to 0.63 after 32 days (Figure 2).
Start-up Stage
In this stage, the physicochemical parameters were assessed to observe the operation of the UASB system and the formation of biofilm of the bacterial consortium. Regular measurements were made for this stage up to 14 days. The temperature values show a maximum of 21 °C. There is a decrease in the pH values from 6.41 to 6.23; in the COD from 2,960 to 960 mg/l; in the total dissolved solids from 1,900 to 800 mg/l; in suspended solids from 370 to 179 mg/l; and in the turbidity from 463 to 234 (FAU) after 14 days (Figure 3).
Operating Stage of the UASB Reactor
In this stage, the physicochemical parameters were measured for the monitoring of the UASB system and the removal of the brl direct blue dye. Regular evaluations were made for this stage up to 28 days.
The pH is 7 up to 28 days, with the pH values of the effluent presenting the lowest values from 7 to 28 days with a value of 6.33 in the last measurement. The reactor's temperature values recorded the highest values between 14 and 28 days with 22.5 °C in the last measurement with a COD removal percentage of 57% from 484 mg/l to 122 mg/l after 28 days in the effluent of the UASB system with activated carbon. The values of the total dissolved solids (TDS) up to 28 days present the lowest values of TDS of the effluent and the reactor compared to the TDS of the feeder, reaching a value of 125 mg/l in the effluent for the last measurement. The lowest values of suspended solids (SS) of the effluent and the reactor compared to the SS of the feeder throughout the operating stage present a value of 104 mg/l in the effluent for the last measurement. The lowest values of turbidity of the effluent and the reactor compared to the turbidity of the feeder through the operating stage reached a value of 130 FAU in the effluent for the last measurement (Figure 4).
In the operating stage of the UASB reactor up to 28 days, the ANOVA statistical test of comparison shows highly significant differences (p < 0.01) with a 99% confidence level in the residual concentration of brl direct blue dye at the different measurement times in the components of the UASB reactor.
Removal of Brl Direct Blue Dye in the UASB Reactor
The lowest concentrations of the brl direct blue dye occurred in the effluent of the UASB reactor compared to the concentration of the dye in the reactor bed at a confidence level of 99%, up to 28 days. This presented a degradation of the dye from an initial concentration of 69.61 mg/l to 9 mg/l after 28 days (Figure 5.E), representing 87% removal of brl direct blue dye.
In Figure 6, a progressive decrease in the concentration of brl direct blue dye is observed, the concentrations being lower up to 9 mg/l in the effluent compared to the reactor up to 28 days.
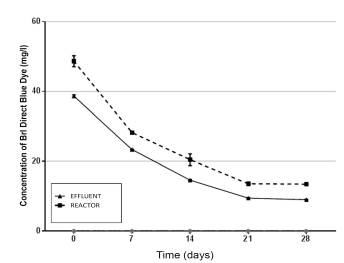
Figure 6 Residual concentration of the brl direct blue textile dye in the operating stage of each component of the UASB reactor according to evaluation time.
In Figure 7, a greater percentage of removal is observed in the effluent of the UASB system until the last measurement. The initial removal was 44.76%; after 7 days, the removal was 66.67%; after 14 days, it was 79.13%; after 21 days, it was 86.51%; and finally, there was 87.14% removal of the dye after 28 days.
Removal of Brl Direct Blue Dye in the Column with Granular Activated Carbon
The residual concentration of brl direct blue dye with just the effect of granular activated carbon presented a 61% removal with values from 70.67 mg/l to 27.83 mg/l. This indicates that the activated carbon used in the adsorption tests presents medium efficiency in the removal of the dye, because compared to the results reported by Conceição (2013) for indigo blue of 99.6% for 150 mg/l of activated carbon, it is much less. This may be due to the different composition of the dye.
Discussion
An increase in volatile suspended solids from 12,894 to 21,546 mg/l is reported in the cultivation stage, higher than the amount reported by González & Escamilla (2006), who took a sample of used activated carbon and established that the content of volatile suspended solids was 159.08 mg/l, which corresponds to the amount of biomass that attaches to the carbon as biofilm. This indicates adequate formation of the biofilm of anaerobic bacteria from the industrial effluents under the conditions of the experiment, because they have reported obtaining bacterial or microbial consortia capable of removing the dyes due to the adaptation of the organisms, the environmental stress and the evolutionary pressure because of the effluent's conditions (Yu et al. 2001; Dafale et al. 2008; Kalyani et al. 2008).
In the operating phase, a 57% removal of the COD is shown, from 484 mg/l to 122 mg/l after 28 days, similar to the value reported by González & Escamilla (2006) in the removal of 100 mg/l of reactive red dye up to 20 days, which was 56%. This is because high proportions of biomass in the water cause an increase in the COD and inhibit the effect of interaction between activated carbon and the microorganisms (González & Escamilla, 2006).
The removal percentage of the brl direct blue dye obtained in the UASB reactor with activated carbon after 28 days was 87%, above the value reported by González & Escamilla (2006) in a UASB system for the biodegradation of 100 mg/l of red reactive dye up to 20 days in which they achieved an 85% removal of the dye. However, both removal percentages were lower than those obtained by Toorisaka, et al. (2005), who obtained 90% efficiency in decolorization in the UASB system with 0.627 mmol/l of methlylene blue dye, demonstrating that it helps to increase the reduction of the dye due to the interaction between the activated carbon and the microorganisms. Toorisaka, et al. (2005) report a lower concentration of 45 mg/l in the COD. The important effect of the activated carbon is because the first stage of dye removal is adsorption over activated carbon and biosorption over the cells of the consortium. As well as the start of the biochemical reaction, by using a greater amount of activated carbon, there is a greater surface for adsorption and also for the formation of biofilm. However, Conceição et al. (2013) obtained a 97% removal of 300 mg/l of indigo blue dye using ceramic clay as an adsorbent material, with a concentration of 200 g/l. This would indicate that optimum results are achieved when using ceramic clay compared to activated carbon, and that it can be used as an adsorbent material such as a treatment post-unit for the elimination of dyes. Sponza and IŞik (2005) in their work on Direct Black 38 dye in anaerobic conditions obtained a 97% efficiency in decolorization and 70% efficiency in the removal of the COD. For the anaerobic decolorization of reactive dye effluents using a UASB system with cassava as a co-substrate, Chinwetkitvanich et al. (2000) obtained a 63% efficiency in decolorization. In decolorization of azo dyes in a UASB system, Brás et al. (2005) obtained a 85-92% efficiency with 92% removal of the COD in 60 mg/l of dye. Içik and Sponza (2005) conducted work on the effect of alkalinization in the UASB system through the decolorization of Congo red azo dye, obtaining total decolorization efficiency and 82-90% removal efficiency of the COD. In a study on the treatment of waste water with textile dyes using the UASB system on waste water with sago as a co-substrate, Senthilkumar et al. (2011) obtained 91.3% decolorization and a maximum removal of the COD of 88.5%. In a combined aerobic/anaerobic treatment used to treat textile industry waste water using the UASB system, O'neill et al. (2000) obtained 88% removal of the COD and a maximum decolorization of 70%. While in a study on the decolorization of commercial azo dyes, Manu & Chaudhari (2002) obtained a maximum removal of the COD of 95% and decolorization of more than 99%, in their work on biodegradation of the color of azo dye (reactive Black 5, RB 5), Karataç and Dursun (2007) obtained full decolorization of 99%.
The chemical structures of the dyes are often too complex to use a simple treatment. Therefore, microbial consortia are generally used with the capacity to degrade dyes, obtaining high purification efficiencies. Many of these consortia are not completely characterized and the mechanism that carries out the degradation is unknown. The development of these technologies is based on conventional techniques without taking into account that the biodegradation activity of a group of organisms does not just depend on a single species, but it is generally the result of collective action of the metabolic diversity present in the medium (Cortazar et al. 2012).
The pH value of the effluent after 28 days was 6.33, presenting an 87% removal percentage of brl direct blue dye within the removal range obtained by González & Escamilla (2006) for Lanasol Red dye, who obtained removal percentages of the COD from 54 to 93% in the study without a pH control. However, the removal percentage in this same research with a pH control at 5 was between 85.62 and 96.79%.
The residual concentration of brl direct blue dye with the effect of the activated carbon of 125 mg/l presented a 61.37% removal with values from 70.67 mg/l to 27.83 mg/l. This indicates that the activated carbon used in the adsorption tests presents a medium efficiency in the removal of the dye compared to the work of Conceição (2013) for indigo blue of 99.6% for 150 mg/l of activated carbon.











 text in
text in 






