INTRODUCTION
Currently, passion fruit crops in Colombia are established from coastal areas up to 1500 m a.s.l., with about 10 000 ha and an approximate production of 165 000 tons per year-1 (Agronet, 2022). The Meta, Antioquia, Huila and Valle del Cauca departments concentrate 63% of the cultivated area and the highest yields (MADR, 2021). Despite this potential, yellow passion fruit crops are being affected by different insect pests and pathogens that decrease yields and reduce fruit quality, making the use agrochemicals necessary (Ocampo et al. 2021).
Pathogens that reduce passion fruit quality and crop yields include plant-parasitic nematodes, fungi, bacteria, and viruses (Ortiz-Paz et al. 2012; Ocampo Pérez et al. 2022). Viruses can reduce the shelf life of passion fruit and affect plant growth, decreasing production by more than 80% (Vaca-Vaca et al. 2016; Asande et al. 2023; Kiptui et al. 2020; Ramos-González et al. 2020).
Currently, five virus genera have been reported affecting the yellow passion fruit crop in Colombia: Potyvirus, Cucumovirus, Tymovirus, Begomovirus and Cilevirus (Benscher et al. 1996; Chavez et al. 1999; Morales et al. 2001; 2002; Vaca-Vaca et al. 2017; Roy et al. 2023).
Soybean mosaic virus (SMV) belongs to the genus Potyvirus, family Potyviridae and species Soybean mosaic virus. It has a positive-sense, single-stranded RNA (+ssRNA) genome of 9,583 nt, a viral protein genome-linked (VPg) covalently linked to the 5' end and a poly-A tail at the 3' end (Zhang et al. 2009; Jaramillo et al. 2018). Several aphid species, including Aphis gossypii and Myzus persicae were recognized as non-persistent transmitters of this virus, which also spreads through seeds (Domier et al. 2007; Jossey et al. 2013; Hajimorad et al. 2018). In Passiflora, SMV causes chlorosis, leaf mosaic and epinasty in early stages of infection, followed by leaf hardening, defoliation and even premature death of infected plants (Benscher et al. 1996).
Cucumber mosaic virus (CMV) belongs to the genus Cucumovirus, family Bromoviridae and species Cucumber mosaic virus. It contains a positive-sense, single-stranded RNA (+ssRNA) genome divided into three segments (RNAs 1, 2, and 3), and is transmitted by seeds and by more than 80 species of aphids (Aphis gossypii among the most effective) in a non-persistent manner (Palukaitis et al. 1992; Chandankar et al. 2013; Cardona et al. 2022a). The characteristic symptoms of this virus consist of a bright yellow mottling on the leaves, which is more intense on leaves close to the site of infection (Gioria et al. 2002). Likewise, roughness and chlorosis of leaves and bleaching of fruits have been associated with the initial infection of this virus in Passiflora (Lan et al. 2020).
Passion fruit leaf distortion virus (PLDV) belongs to the genus Begomovirus, family Geminiviridae and species Passion fruit leaf distortion virus. It contains a single-stranded circular DNA (ssDNA) genome consisting of two components: DNA-A of 2,600 nt and a DNA-B of 2,572 nt (Vaca-Vaca et al. 2017). It is transmitted through the biological vector whitefly (Bemisia tabaci complex) and, in Passiflora, causes symptoms such as yellow mosaic and deformation of leaves and fruit (Vaca-Vaca et al. 2016; 2017).
Passion fruit yellow mosaic virus (PFYMV) belongs to the genus Tymovirus, family Tymoviridae and species Tymovirus passiflorae. It contains a positive-sense, single-stranded RNA (+ssRNA) genome of 6,088 nt and its natural transmission mechanism has not yet been identified. However, its experimental infection has been achieved through mechanical transmission and by the chrysomelid beetle Diabrotica speciosa, exclusively affecting plants of the genus Passiflora, causing symptoms such as yellow nets, bright yellow mosaic and leaf roughness (Crestani et al. 1986; Jaramillo et al. 2019).
Plants are commonly affected by several viruses simultaneously, and understanding the interactions between these causal agents is critical to comprehending viral pathogenesis, which in turn is essential to developing efficient management strategies (Syller, 2012). Plant viruses co-infecting the same host can interact synergistically, causing more severe symptoms than when a single infection occurs (Lamichhane & Venturi, 2015), or antagonistically, when a low virulence virus strain prevents or reduces subsequent infection by a homologous virus (Syller, 2012; Chávez-Calvillo et al. 2016). It has been observed that, in some cases, the outcome depends on the order in which infection occurs (Chávez-Calvillo et al. 2016; Riska & Hisashi, 2020).
A previous report on virus in yellow passion fruit crops in Colombia revealed the presence of potyvirus in samples from the central west of the country between the departments of Valle del Cauca and Risaralda, which was identified as SMV by serological tests (Benscher et al. 1996). In other study, Chavez et al. (1999), using serological tests, identified the presence of potyvirus (SMV strain) and tymovirus in yellow passion fruit leaf samples collected in nine departments of Colombia. Subsequently, these viruses were identified by molecular techniques as SMV and PFYMV (Morales et al. 2001; 2002). Vaca-Vaca et al. (2016) reported for the first time the incidence of begomovirus in yellow passion fruit crops in Valle del Cauca. This begomovirus was characterized at the molecular level, being different from other begomoviruses reported worldwide, and was named PLDV because of the symptoms it causes in passion fruit (Vaca-Vaca et al. 2017). Recently, Roy et al. (2023) identified SMV in a passion fruit leaf sample from the department of Meta and detected Passion fruit green spot virus (PfGSV), a Cilevirus transmitted by mites of the genus Brevipalpus in a leaf sample from the department of Casanare.
The objective of this paper was to analyze the prevalence and identify the types of mixed infections among viruses belonging to the genera Potyvirus, Tymovirus, Cucumovirus and Begomovirus in the main producing areas of yellow passion fruit in Valle del Cauca. The results obtained provide an updated perspective on the viral diversity limiting passion fruit production in Valle del Cauca.
MATERIALS AND METHODS
Collection of plant material. During 2017, a collection of yellow passion fruit leaves with viral symptoms was conducted in six municipalities of Valle del Cauca in Colombia. Among five and ten plants with viral symptoms were randomly collected in each crop. The sampling sites were geo-referenced, and the samples were transported in a styrofoam cooler with cooling gel bags at ≈ 10°C to the Laboratorio de Sanidad y Microbiología Agrícola de la Universidad Nacional Sede Palmira for further processing and molecular analysis. Once in the laboratory, each sample composed of 3 leaves of normal size (≥ 10 cm in length) or 4 leaves of smaller size, was stored at room temperature (25°C) with silica gel, in plastic bags with hermetic seal, thus achieving dehydration and stabilization of the RNA for at least 6 months (Munguti et al. 2016; Ruiz-Vargas et al. 2024).
Purification of nucleic acids.
Extraction and Visualization of Genomic DNA. From each sample, 20 to 30 g of dry leaf tissue was taken and macerated with liquid nitrogen in a mortar until a fine powder was obtained. The powder obtained was transferred to a 1.5 ml microtube and genomic DNA extraction was performed using the cetyl trimethyl ammonium bromide (CTAB) methodology reported by Doyle et al. (1990). Quantification and verification of the quality of the extracted DNA was performed by 0.8% (w/v) agarose gel electrophoresis, which was run in TAE 1X at 70 volts for 50 minutes. Ethidium bromide staining (10 ng/μl) was used, and the Thermo ScientificTM GeneRuler 1Kb DNA Ladder Molecular Weight Marker was used as a reference standard. Development was performed on BioRad Gel Doc XR+ imaging system, consisting of a transilluminator and Quantity One v.4.6.5 software.
RNA Extraction and cDNA Synthesis. Total RNA extraction was performed from each sample using TRIzol→ reagent (InvitrogenTM) following the protocol established by the manufacturer. The determination of RNA purity and concentration was performed on 0.8% agarose gels. From the total RNA extracted from each sample, complementary DNA (cDNA) was synthesized via reverse transcription (RT) using the RevertAid First Strand cDNA Synthesis Kit (Thermo ScientificTM) following the manufacturer instructions.
Virus diagnosis.
DNA Virus Diagnosis. Begomovirus and PLDV were detected from the purified total DNA by Polymerase Chain Reaction (PCR). Universal primers MP16 and MP82 were used, which amplify a fragment between 400-500 bp of the N-terminal region of the capsid protein (Cp) (Umaharan et al. 1998). In the samples that tested positive, specific detection of PLDV was performed using the species-specific primers PLDV-F23 and PLDV-R405 that amplify a 450 bp fragment of Cp (Vaca-Vaca et al. 2020). The amplified products were visualized on 0.8% agarose gels.
RNA Virus Diagnosis. From the cDNA synthesized from each sample collected, cucumovirus, CMV, potyvirus, SMV and PFYMV were detected by PCR using universal primers by viral genus and specific viruses. To detect viruses of the genus Cucumovirus, the universal primers CMV-F 5'- and CMV-R 5' that amplify a 586-bp fragment of the Cp gene were used (Herrera-Vásquez et al. 2009). To detect potyvirus, the universal primers NIb2F and NIb3R that amplify a 350 bp fragment of the NIb (nuclear inclusion protein b) gene were used (Zheng et al. 2010). Cucumovirus-positive samples were evaluated for CMV with the species-specific primers CMV cp-F and CMV cp-R that amplify a 229-bp fragment of the Cp gene, designed by Rivera-Toro et al. (2020) for the detection of CMV in chili peppers (Capsicum sp.). Potyvirus-positive samples were evaluated with SMV species-specific primers designed in this research work: SMV F269 (5´-TGG-GTG-TGG-TTA-TGA-ATG-G-3´) and SMV R562: (5'-CTG-TTT-GGT-GTT-GTT-TTG-GAA-GT-3'), which amplify a 313 bp fragment of the Cp gene. Finally, to detect PFYMV, the following species-specific primers were designed and used: PFYMV-R536 (5'-AAC-CCA-AAC-GAG-AGA-GAC-3') and PFYMV-F294 (5'-AAA-CTC-CCA-TCA-ACA-CCA-A-3'), which amplify a 260 bp fragment of the Cp gene. The amplified products of each virus were visualized on 0.8% agarose gels.
Species-specific Primer Design. The nucleotide sequences of SMV (GenBank KY249373.1) and PFYMV (GenBank AF467107.1) reported by Jaramillo et al. (2018) and Morales et al. (2002), were used to design the species-secific primers for SMV and PFYMV respectively. The design was performed with the CLC Main Workbench 7.9 software. (Qiagen®) and its specificity was validated using the Primer Blast program (Ye et al. 2012).
Incidence Analysis. The incidence percentage (positive samples/total samples*100) of the different viruses (Agrios, 2005) was calculated by locality and municipality. The distribution of single, double and triple infections in the different municipalities was determined. The correlation between the altitudinal gradient 924 - 1692 m a.s.l., and the presence of viruses was evaluated by Pearson correlation (cor.test function, Stats R package) (R Core Team, 2013). The correlation between this altitudinal gradient and the presence of single, double and triple infections was also estimated.
RESULTS AND DISCUSSION
Detection of begomovirus and PLDV in yellow passion fruit crops. A total of 66 passion fruit leaf samples at different phenological stages were collected. The discrimination of the samples by municipality is shown in Table 1. Of the total number of samples analyzed, 45.5% were positive for begomovirus. In general, begomoviruses were distributed in five of the six municipalities evaluated, and in four of them they presented an incidence higher than 50% (Table 1).
The localities with the highest incidence of begomovirus belong to the municipalities of El Cerrito and Bolivar. In contrast, the samples from the municipality of La Unión were the only ones found to be free, not only of begomovirus, but also of the other three RNA viruses evaluated in this research: cucomovirus, potyvirus and tymovirus (Table 1).
Table 1 Incidence of viral infections in yellow passion fruit crops in Valle del Cauca, Colombia.
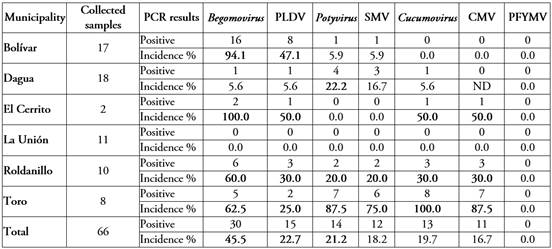
Incidences greater than 20% in each municipality are indicated in bold. ND, not determined. PLDV: passionfruit leaf distortion virus, SMV: soybean mosaic virus, CMV: cucumber mosaic virus, PFYMV: passion fruit yellow mosaic virus.
Of the begomovirus-positive samples, 50% were found to correspond to PLDV, a virus previously detected in yellow passion fruit crops in the municipalities of Palmira and La Unión, in the department of Valle del Cauca (Vaca-Vaca et al. 2016; 2017). Fifteen of the 30 samples in which begomovirus was detected gave negative results when evaluated by PCR for PLDV. This suggests that there is another virus or viruses of this genus infecting yellow passion fruit crops in the department.
Detection of potyvirus, SMV, cucumovirus, CMV and PFYMV in yellow passion fruit crops.
(a) Potyvirus and SMV. Of 66 samples analyzed, 21.2% were found to be infected with potyvirus. Table 1 shows the diagnosis by municipality. Of these samples, 85.7% were identified as SMV using specific primers. In addition, 2 of the 14 samples in which the presence of virus of the Potyvirus genus was detected gave negative results when evaluated by RT-PCR for SMV, which suggests the presence of another virus of this genus in passion fruit crops in Valle del Cauca. The highest incidence of potyvirus was observed in yellow passion fruit crops located in the municipality of Toro, followed by the municipalities of Dagua and Roldanillo. Finally, samples from crops located in the municipalities of El Cerrito and La Unión were free of infestation by pathogens of this viral genus (Table 1).
In general, potyviruses were identified in four of the six municipalities included in this study, with an incidence higher than 20% in three and an incidence of 87.5% in one. In a study reported by Benscher et al. (1996) it was indicated that SMV was detected in three species of the Passifloraceae family in the departments of Valle del Cauca and Risaralda: passion fruit (P. edulis f. flavicarpa Degener), granadilla (P. ligularis Juss.) and badea (P. quadrangularis L.). Subsequently, in other reports and in this study, the prevalence of this pathogen has been recorded in yellow passion fruit crops in the department of Valle del Cauca for at least 26 years (Benscher et al 1996; Morales et al. 2001). Likewise, SMV was detected in passion fruit plants in the department of Meta (Roy et al. 2023). The persistence of this virus in yellow passion fruit and other passion fruit crops in this department and, in general, in the country, is explained by the ability of this virus to be transmitted to the following generations by seed (Domier et al. 2007; Cardona et al. 2022a;b) and by the use of seeds of unknown phytosanitary quality by farmers, due to a shortage of certified nurseries for obtaining virus-free seeds (Rodríguez et al. 2016).
(b) Cucumovirus and CMV. PCR analysis detected the presence of cucomovirus in 19.7% of the total samples analyzed and the distribution in the different municipalities are shown in Table 1. Of these samples, 84.6% amplified the CMV capsid protein gene fragment. Additionally, 2 of the 13 samples in which the presence of virus of the genus Cucumovirus was detected gave negative results when evaluated by PCR for CMV. This suggests that there is another virus or viruses of this genus infecting passion fruit crops in the department.
The highest incidence of cucumovirus was observed in yellow passion fruit crops located in the municipalities of Toro and Roldanillo. In contrast, samples from the localities tested in the municipalities of Bolívar and La Unión were free of this viral pathogen (Table 1). Possibly, the crops sampled in these localities were initiated with cucumovirus-free plant material, and adequate management of aphids and the natural vector of this virus was done (Chandankar et al. 2013; Cardona et al. 2022a).
In general, cucumoviruses were identified in four of the six municipalities included in this study. Three of these had an incidence higher than 30% and one had an incidence of 100%. In previous studies, CMV was found in gulupa (P. edulis f. edulis Sims) crops in Antioquia (Cardona et al. 2022a;b).
(c) Tymovirus PFYMV. Of the 66 samples analyzed, none amplified by PCR the expected 262 bp fragment for the detection of tymovirus PFYMV (Table 1). Crestani et al. (1986) reported by serological assays (ELISA) the presence of a virus of the Tymovirus genus, PFYMV, which was detected in Brazil affecting yellow passion fruit crops. In Colombia, Morales et al. (2001) reported the detection and partial genome sequence of a virus like the PFYMV (pathogenically and antigenically) in yellow passion fruit crops located in the departments of Antioquia, Caldas, Santander and Valle del Cauca. Recently, Jaramillo et al. (2019) published the complete genome of the PFYMV virus affecting passion fruit in Colombia, warning that the partial sequence of PFYMV (AF467107.1) reported in the GenBank database by Morales et al. (2001), showed some errors at the 3' end, specifically in the ORF coding for the capsid protein. This could explain the negative results obtained in this study, since the primers designed in this research work were created on the region having errors, i.e. the PFYMV capsid protein gene reported by Morales et al. (2001).
It is suggested to design new primers on the new sequence reported by Jaramillo et al. (2019) or use those already elaborated by these authors to conduct future PFYMV detection studies in passion fruit crops. In the department of Antioquia, Cardona et al. (2022a;b) documented the presence of this virus in both symptomatic and asymptomatic samples of gulupa, with an incidence ranging from 30 to 90%. This finding was achieved by amplification of the aforementioned primers Tymo_F_CP and qTymo_R_CP designed by Jaramillo et al. (2019).
Mixed infections of three viral genera in yellow passion fruit crops.
Plant diseases are commonly the result of interactions between complexes of microorganisms, and hardly of isolated pathogens (Lamichhane & Venturi, 2015). In this investigation, null infections (no infections detected), single (a single virus genus identified), and mixed infections by two (double infections) and three (triple infections) virus genera were detected in the same sample.
Table 2 shows the incidence of mixed infections in 66 samples collected in passion fruit crops, where begomoviruses (PLDV) have the highest incidence in single infections (28.8%), followed by cucumoviruses and potyviruses. At the level of double infections, an incidence of 6.1% can be observed for the combinations Potyvirus + Cucumovirus and Potyvirus + Begomovirus, while the combination Cucumovirus + Begomovirus had an incidence of 4.5%. Finally, at the level of triple infections, the simultaneous presence of the three viral genera, Potyvirus, Cucumovirus and Begomovirus, was determined in four of the samples evaluated, confirming the joint presence of SMV, CMV and PLDV. This highlights the high variability of interactions that occur naturally in agroecosystems, useful information for the management of viral diseases in plants (Syller, 2012).
Multiple infections were observed in five of the six municipalities evaluated (Table 2). In municipalities such as Bolivar, Dagua, El Cerrito, and Roldanillo, both single and double infections were observed. In the municipality of Toro, only double and triple infections were found, and it was the only municipality where triple infections were detected (Table 2). Such unpredictable pathogen-induced biological and epidemiological effects are the result of constant battles between the genes of the different viruses and the host plant (Liang et al. 2016). Likewise, the municipality of La Unión stood out because none of the viral genera included in this study were detected in the samples from the two localities of this municipality included in this study (Table 2). Therefore, it is suggested that the visualized symptoms ranging from leaf lamina chlorosis, interveinal chlorosis, epinasty, yellow mosaic, to leaf roughness and deformation, in the crops sampled in these two localities, may be due to nutritional deficiencies or other stress factors in the plant (Datnoff et al. 2007; Hull, 2009).
Table 2 Types of mixed viral infections identified in yellow passion fruit crops in Valle del Cauca, Colombia.
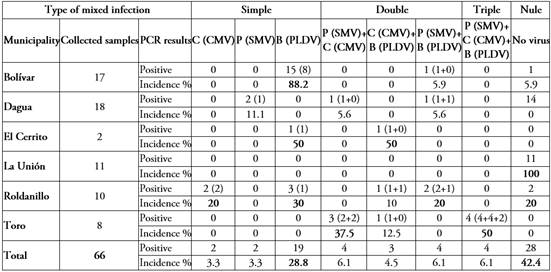
Incidence greater than 20% is indicated in bold. Nomenclature for viral genera: Cucumovirus (C), Potyvirus (P) and Begomovirus (B). The number of samples that tested positive for the specific viruses is indicated in parentheses: C (CMV), P (SMV) y B (PLDV).
The main symptoms observed in passion fruit samples positive for virus infections were leaf roughness and deformation (LRD), epinasty (EPI), chlorosis (C) and yellow mosaic (YM) (Figure 1). The samples in which a greater number of symptoms were observed corresponded to simple viral infections by begomovirus (Figure 2c).
Additionally, greater symptom severity was observed in samples in which mixed infections, both double and triple, were detected (Figure 2), suggesting the existence of synergistic interactions (Lamichhane & Venturi, 2015). These results are consistent with the study conducted by Riska & Hisashi (2020) in which mixed artificial inoculation of East Asian passifora virus and East Asian passifora distortion virus belonging to the genus Potyvirus was performed on Passiflora foetida plants, and it was observed that the severity of symptoms after double inoculation was greater than that observed after single infection with either of the two potyviruses. On the other hand, Syller (2012) reported CMV in synergistic co-infections with a potyvirus, potato virus Y (PVY) and suggested that the outcome of interactions between potyviruses and other viruses depends on the co-evolution between these and the host plant.
On the other hand, no significant correlation (p > 0.05) was found in this study between the viral infections (genera and species) detected and the altitudinal range where the infected samples came from (data not shown). There was also no significant correlation between the altitudinal range of the samples and the type of infection detected in the samples: single, double and triple infections (data not shown).
Relationship between the observed symptomatology and the type of virus infection detected in yellow passion fruit crops. The 93.3% of the samples showed typical symptoms of virus infection (Figure 1) such as bulging (B), chlorosis (C), interveinal chlorosis (iC), defoliation (D), deformations (DEF), dwarfing (Dw), epinasty (EPI), yellow mosaic (YM), leaf roughness and deformation (LRD), and fruit roughness and deformation (FRD). A total of 6.7% of the samples showed no symptoms.
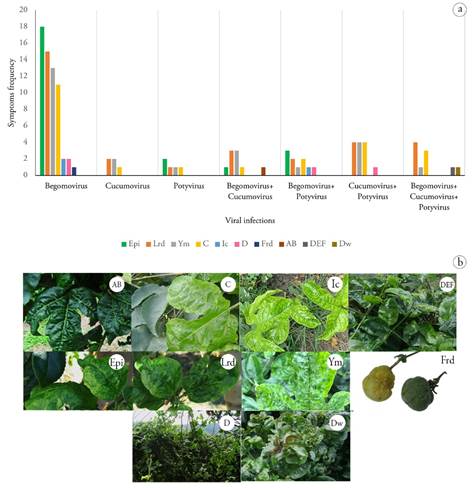
Figure 1 a) Symptoms frequency associated with single, double and triple viral infections b) photography of symptoms: AB (Bulging), C (Chlorosis), Ic (Interveinal chlorosis), D (Defoliation), DEF (Deformities), Dw (Dwarfism), Epi (Epinasty), Ym (Yellow mosaic), Lrd (Leaf Roughness and deformation), Frd (Fruit Roughness and deformation).
In the samples with single Begomovirus infections (Figure 2), symptoms such as: epinasty, leaf roughness and deformation were observed in the 19 positive samples, yellow mosaic in 14 samples, chlorosis in 11 samples, interveinal chlorosis in three samples, and defoliation in two samples. Of the two samples in which single Potyvirus infection was detected, epinasty was observed in two, and chlorosis, yellow mosaic, roughness and fruit deformation were also observed in one of them. In the two samples with simple infection by Cucumovirus, yellow mosaic, roughness and deformation of leaves were observed, and in one of them chlorosis was also observed. In the four samples with double Potyvirus + Cucumovirus infection, chlorosis, yellow mosaic, roughness and leaf deformation were observed, and defoliation was also observed in one of them. In the three samples in which double Cucumovirus + Begomovirus infection was identified, roughness and deformation of leaves and yellow mosaic were observed, in one there was also chlorosis and in another there were also epinasty and bulging. Of the four samples in which double Potyvirus + Begomovirus infection was detected, one was asymptomatic, three showed epinasty, two showed chlorosis, two showed leaf roughness and deformation, one showed interveinal chlorosis, and one showed defoliation. In the four samples in which triple Potyvirus + Cucumovirus + Begomovirus infection was detected, roughness and leaf deformation were observed. Chlorosis was also observed in three of them, dwarfing was observed in one sample, and yellow mosaic and deformations were also observed in another. Fruit deformation was observed in one sample.

Figure 2 Symptomatology observed in yellow passion fruit plants with different viral infections. Simple infections: a) Cucumovirus (CMV); b) Potyvirus (SMV); c) Begomovirus (PLDV). Double infections: d) [Potyvirus (SMV) + Cucumovirus (CMV)]; e) [Cucumovirus (CMV) + Begomovirus; f) [Potyvirus (SMV) + Begomovirus. Triple infections: g and h) [Potyvirus (SMV) + Cucumovirus (CMV) + Begomovirus] and i) [Potyvirus (SMV) + Cucumovirus (CMV) + Begomovirus (PLDV].
Other authors have also associated different symptoms with the viral infections detected, such as Benscher et al. (1996) who observed yellow mosaic, epinasty and defoliation in passion fruit samples inoculated with SMV potyvirus. Likewise, Chavez et al. (1999), when inoculating potyvirus and tymovirus in different plants, observed that: (a) in Passifloras, interveinal chlorosis, yellow mosaic, chlorosis, deformations, (b) in beans (Phaseolus vulgaris), necrosis of veins, roughness and deformation of leaves, and yellow mosaic, and (c) in Physalis floridiana interveinal chlorosis. Morales et al. (2001) reported the identification of Potyvirus SMV causing deformations, yellow mosaic and ringed spots, and tymovirus PFYMV causing yellow mosaic with a tendency to form bands on the leaves. Vaca-Vaca et al. (2017) found yellow mosaic and leaf and fruit roughness and deformation in samples diagnosed with Begomovirus PLDV.
In conclusion, the prevalence of viruses affecting yellow passion fruit crops in the main producing municipalities of the department of Valle del Cauca was determined. The presence of single, double and triple infections was established among viruses of the Potyvirus, Cucumovirus and Begomovirus genera, associated with symptoms such as roughness and deformation of leaves, yellow mosaic, chlorosis, epinasty, roughness and deformation of fruits. In symptomatic samples with double and triple infections, a greater severity of symptoms was apparently observed, suggesting synergistic interactions between the viruses involved.
The results obtained in this study indicate that begomovirus PLDV prevails over SMV and CMV as a viral agent that is limiting passion fruit production in Valle del Cauca. This information is quite useful for the producer, since it allows him not only to orient his cultural activities towards the control of these phytopathogens but also to undertake future breeding programs aimed at obtaining materials tolerant to viruses that really affect and limit the production of this Passiflora in Valle del Cauca.















