INTRODUCTION
Macrophytes exhibit macroscopic forms of aquatic vegetation and serve as a link between water and sediment. Angiosperms, ferns, macroalgae and mosses found in both lotic and lentic aquatic ecosystems belong to this group (Regmi et al. 2021). They also act as indicators of the ecological conditions of surface waters, since they are primary producers of aquatic habitats, which serve as an important component in the functioning of the ecosystem, from the maintenance of biotic and abiotic factors (Upadhyay et al. 2022).
Macrophytes play an important role in structuring aquatic environments, especially those of lentic type, since their presence in these ecosystems increases the complexity of the habitat, significantly influencing aspects such as diversity, distribution, richness, and abundance of the aquatic macroinvertebrates (Heisi et al. 2023). These plants increase the structural heterogeneity of macroinvertebrates by providing refuges against predators (Misteli et al. 2022), offering food for herbivores and detritivores, (Fontanarrosa et al. 2013) and providing spaces that facilitate emergence and oviposition (Walker et al. 2013).
Within macroinvertebrates, aquatic insects are distinguished by presenting a high number of organisms that make up the most significant component of animal biomass in lotic and lentic ecosystems. Their contribution to the food web is relevant, because they allow energy circulation to other consumers, serving as food for fish and amphibians and becoming an important link between producers and the higher trophic levels of aquatic ecosystems (Walteros & Castaño, 2020).
In Colombia, a series of investigations have been conducted on aquatic macroinvertebrates associated with macrophytes and the most recent to be highlighted are those conducted by Rúa-García (2015), Hernández et al. (2016), Murillo-Montoya et al. (2018), and Nuñez & Fragoso-Castilla, (2019). In the department of Chocó, on the topic of aquatic macroinvertebrates associated with macrophytes in wetland ecosystems, the research conducted by Mosquera-Murillo & Córdoba-Argón (2015); Mosquera-Murillo (2018), and Aguilar-Baldosea et al. (2022) is notable. However, further research is needed to continue contributing to the understanding of the dynamics of these communities.
Therefore, the objective of this research is to determine the composition, diversity, and abundance of aquatic entomofauna associated with macrophytes in wetlands of the middle basin of Atrato River (Chocó-Colombia), as well as to identify the functional groups of insects associated with macrophytes, in addition to establishing the possible relationship between them and the abiotic variables of the wetlands studied.
MATERIALS AND METHODS
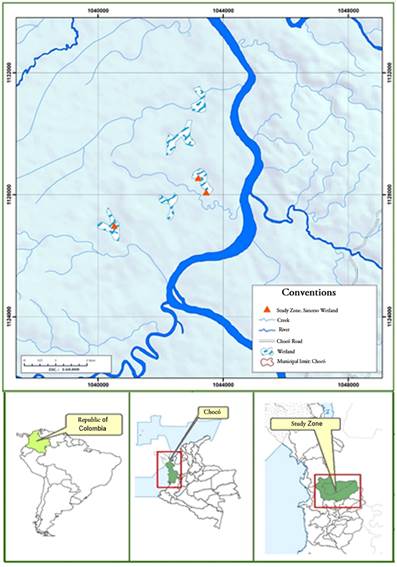
Scope of study. The research was carried out in three wetlands in the middle basin of Atrato River, belonging to the municipality of Quibdó, Chocó-Colombia: Plaza Seca wetland 5°44’32.3”N 76°42’37.7”W, La Negra wetland 5°45'30.9"N, 76°41'14.7"W, and La Grande wetland 5°44'39.8"N, 76°42'42.1"W (Figure 1). This zone experiences a relative humidity close to 86% and temperatures that range between 28-32°C. The majority of the territory lies within the equatorial doldrums; therefore, rainfall regime extends throughout the year, with an annual precipitation reaching up to 12,000 mm (Rangel-Ch. & Arellano-P., 2004).
According to the Holdridge system (1996), the Atrato River basin in its course is classified into the humid tropical forest and too humid tropical forest life zones (bh-T, bmh-T). The middle and lower zones comprise the floodplain of Atrato River, characterized by vast floodplains and several swamps known for their high transparency, acidic pH, low conductivity, and concentration of solids, as well as low oxygen and few nutrients (Correa, 2014). The dominant macrophyte species in the studied wetlands are Eichhornia azurea, Nymphoides indica, Ludwigia sedoides, Cabomba sp., Elodea sp. (Mosquera & Córdoba, 2015).
Sampling. Monthly sampling was conducted in each of the studied wetlands, between June and September 2022, covering the climatic periods of the zone, the high water season (June-July) and the low water season (August-September).
In each studied wetland, a sampling station was established (coastal zone). Within the macrophyte-covered area, the physical and chemical water conditions were measured, such as water temperature, pH, electrical conductivity and dissolved oxygen concentration, total dissolved solids, and transparency using a multiparameter digital equipment and a Secchi disk. Additionally, water samples were collected for alkalinity, phosphorus, and nitrogen form analyses, following the recommendations of Standard Methods (APHA et al. 2012). Likewise, the forms of phosphorus (orthophosphates) and nitrogen (nitrites, nitrates, and ammonium) were analyzed using a NOVA SQ 60 spectrophotometer.
To collect insects, transepts perpendicular to the shoreline were established in the macrophyte belts. A one m2 floating PVC frame, equipped with a 0.5 mm mesh was used, which was installed under the surface to be collected. Plants were extracted and the roots were carefully washed to remove the organisms. Using a sieve and tweezers, the organisms were extracted and stored in jars with 90% alcohol.
In the limnology laboratory of the Universidad Tecnológica del Chocó (UTCH), organisms were identified to the lowest possible level using a ZEIZZ brand stereomicroscope and the keys from Domínguez et al. (2006) and Domínguez & Fernández (2009). Additionally, each taxon was assigned to a functional group according to the classification proposed by various authors such as Chará-Serna et al. (2010), Rodríguez-Barrios et al. (2011), and Rivera-Usme et al. (2013). The considered groups were predators, collectors, collector-gatherers, collector-filterers, and shredders (Merrit & Cummins, 1996).
Data analysis. The aquatic insect assemblage was characterized based on the following variables: total number of individuals, relative abundance, Shannon-Wiener diversity, Simpson dominance, and classification of food functional groups. Differences in abundance, diversity, and dominance among wetlands were assessed using an ANOVA test. Mean and standard deviation were estimated for each measured abiotic variables, and differences among wetlands were evaluated using ANOVA. Assumptions inherent to the ANOVA test were verified for all cases and found acceptable (p>0.05). Significant differences were recorded using a Tukey test and the Minitab program was used. Finally, the potential relationship between aquatic insects and the abiotic variables of the wetlands was evaluated using a canonical correspondence analysis.
RESULTS AND DISCUSSION
Aquatic insects associated with macrophytes. A total of 654 individuals were registered, distributed in 6 orders, 23 families and 36 genera (Table 1). Plaza Seca wetland had the highest number of individuals (315), followed by La Grande wetland (183), and La Negra wetland (156). The variance test showed significant differences between the wetlands with respect to the abundance of aquatic insects.
Table 1 Taxonomic list and ecological indexes of aquatic insects associated with macrophytes in three wetlands in the middle basin of Río Atrato, Chocó (Chará-Serna et al. 2010; Rodríguez-Barrios et al. 2011; Rivera-Usme et al. 2013).
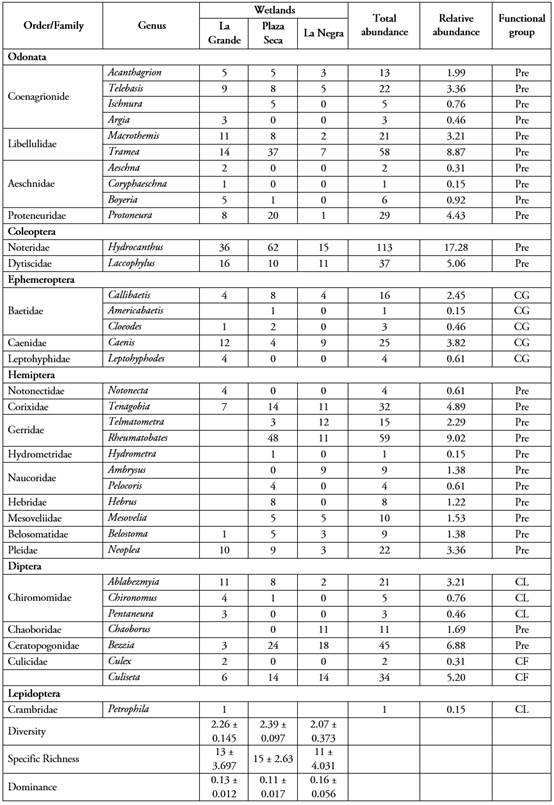
Pre= Predator; CG=Collector-Gatherer; CF= Collector-Filterer; CL= Collector
The presence of aquatic insects associated with floating aquatic macrophytes is associated with the presence of a dense mass of plant roots that constitute colonization sites for different organisms. Root macrophytes provide microhabitats that serve as refuge against predators and for egg-laying, as well as resources as food for fauna (Padial et al. 2009).
Regarding orders, Hemiptera is the most representative in Plaza Seca and La Negra wetlands, while Odonata was the most representative in La Grande wetland (Figure 2). Among the families, Noteridae (16.32 ± 5.81) and Libellulidae (11.24 ± 4.75) were notable. According to Lasso et al. (2014), it is common to find a high representativeness of Odonata-Coleoptera-Hemiptera in lentic ecosystems of the country, as observed in this research. Aquatic and semi-aquatic hemipterans are notable for their variety, reflecting the variety of niches they occupy, thanks to the great adaptation capacity to the environment their species have (Vanegas, 2017). This can be found in a wide variety of natural environments, both lotic and lentic, coastal and oceanic, phytotelmata and even humid terrestrial environments (Mazzucconi et al. 2009).
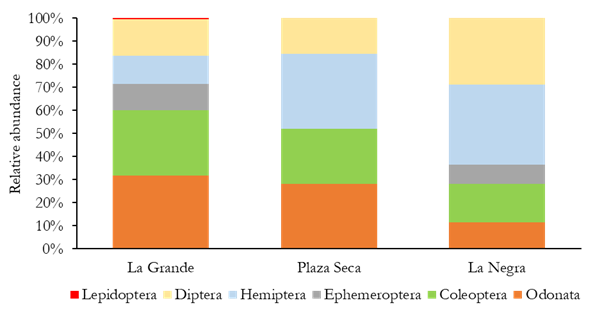
Figure 2 Abundance of specimens of insect orders found in the studied wetlands in the middle part of Atrato River.
As for odonates, their abundance has been positively related to the presence of aquatic plants typical of wetlands since emerging vegetation is a fundamental substrate for the emergence of larvae into their adult phase. Likewise, a high macrophyte coverage is important for the richness of Odonata taxa (Perron et al. 2021). In addition to the existence of aquatic plants, physicochemical characteristics are important factors that limit the richness and distribution of immature odonates (Córdoba-Aguilar, 2008).
The abundance of Noteridae and Libellulidae in this study corresponds to other research carried out in wetlands of the region, such as the one conducted by Mosquera-Murillo & Córdoba-Argón (2015). According to White & Roughley (2008), members of the family Noteridae are common into the roots of aquatic plants and their ability to take atmospheric oxygen makes them dependent on atmospheric oxygen. This is a primary factor that restricts these beetles to shallow waters (Eyre et al. 1992), which agrees with the characteristics of the studied wetlands.
For the Libellulidae, its abundance is associated with the great diversity of emerging rooted macrophytes present in the studied wetlands, which, according to López-Díaz et al. (2021), is a factor that can increase the complexity of the habitat. Nymphs of Libellulidae inhabit all freshwater aquatic environments, including lentic and lotic systems, as well as permanent and temporary environments (Neiss et al. 2018).
Plaza Seca wetland has the highest averages of diversity and specific richness, while dominance showed higher averages in La Negra wetland (Table 1). The analysis of variance shows that there were no significant differences in diversity (F=1.84, p=0.2135) and dominance (F=2.23; p=0.1635) among wetlands, but there were differences in specific richness (F=7.24; p=0.0133). These results may be associated with how aquatic vegetation is distributed in the wetlands under study and, in that sense, research such as that of Warfe et al. (2008) and Dibble & Thomaz, (2009) has shown that the complexity of this type of vegetation explains attributes of the invertebrate assemblages associated with it, such as abundance and diversity. Similarly, according to authors such as Quirós Rodríguez et al. (2010) and Kovalenko et al. (2012), the presence of floating vegetation, as well as the complexity of its architecture, provides a greater variety of environments and microhabitats that constitute a substrate and refuge, leading to greater diversity, richness, and abundance of associated organisms.
In relation to the composition of functional groups of aquatic insects associated with macrophytes, in the three studied wetlands, predators were the largest group with a range among wetlands between 87.94 and 73.77%, with 15 families corresponding to Odonata, Hemiptera and Coleoptera, and certain families of Diptera (Chaoboridae and Ceratopogonidae). They are followed by collector-gatherers with a range between 11.48 and 4.76%, represented by 3 families of Ephemeroptera. Collectors showed a range between 10.38 and 1.28% with two families of Lepidoptera and Diptera (Chironomidae). Collectors-filters had a range between 8.97 and 4.37%, with a family of Diptera (Culicidae) (Table 1).
The greater representativeness of predators may be related to the high structural complexity of the habitat generated by macrophytes (Heino, 2000), which is an indicator of an abundant food supply. Particularly, aquatic vegetation constitutes a refuge zone for invertebrates to avoid predation by fish, therefore representing an important supply for predatory invertebrates whose number can increase when there is more prey (De Neiff & Neiff, 2006).
Environmental conditions of macrophyte habitat and aquatic insect community. Regarding the abiotic variables analyzed in the three studied wetlands, pH, water temperature, and transparency showed significant differences among wetlands (ANOVA, p<0,05), with pH and transparency presenting their highest averages in Plaza Seca wetland, and water temperature in La Grande wetland. The remaining variables did not have significant differences (ANOVA, p>0.05) (Table 2); however, dissolved oxygen, alkalinity, electrical conductivity, and total solids were higher in La Grande wetland. As for nutrients, these tend to be higher in La Grande (Nitrites), Plaza Seca (Nitrates) and La Negra (Orthophosphates) wetlands. Ammonium was not detectable. Depth was greater in La Negra wetland.
Table 2 Abiotic variables of the studied wetlands in the middle part of Atrato River.
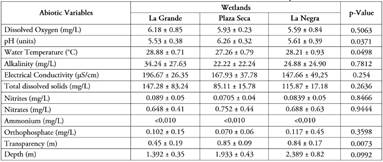
Average values ± standard deviation
Dissolved oxygen and pH variables had values similar to those reported for other wetlands in the Atrato basin, such as the studies conducted by Mosquera-Murillo & Córdoba-Argón (2015) and Mosquera-Murillo (2020). According to Martínez-Rodríguez & Pinilla-A. (2014), one of the wetland characteristics is oxygen deficiency, as a result of slow exchange with air since water turbulence is less than that of a river. As for pH, the recorded values are largely attributable to the nature of the soils in the zone, which are acidic like those of the majority in the department of Chocó, being within the limits for the survival of aquatic organisms that is between 4.5 to 8.5 (Roldán & Ramírez, 2008). Water temperature averages, in turn, are typical of aquatic environments located in tropical regions, with values between 25 and 30°C (Roldán & Ramírez, 2008).
Electrical conductivity, alkalinity and total dissolved solids variables were within the ranges established for Colombian neotropical ecosystems, with values less than 1,500 μS.cm-1, 100 mg.L-1 and 200 mg.L-1, respectively (Roldán & Ramírez, 2008). Transparency is within the values established for wetlands in Colombia (<1.13 m), while depth is associated with variations in the flow of Atrato River, as a result of the climatic regime of the zone. Nitrites were found in low concentrations in the studied wetlands in comparison to nitrates. According to Wetzel (2001), nitrogen as nitrites tends to be low in aquatic systems, since it quickly changes to nitrates depending on the oxygen concentration in water, or to reduced forms such as ammonium if the conditions are anoxia, a situation that may be occurring in the studied wetlands. As for ammonium, the values were extremely low and were below the detection range. Orthophosphates, in turn, had averages above 0.05 mg.L-1, which is the limit required for aquatic life.
According to the canonical correspondence analysis, the first two canonical axes explain 68.32% of the variance. The analysis demonstrates that organisms of the Protoneuridae, Pleidae, Chironomidae and Culicidae showed a positive relationship with electrical conductivity, nitrites, and nitrates and a negative relationship with transparency. Corixidae, Gerridae, and Ceratopogonidae were negatively related to electrical conductivity, nitrites, and nitrates and positively to transparency. Caenidae and Culicidae were positively associated with phosphates and depth, while Dytiscidae showed an opposite relationship (Figure 3).
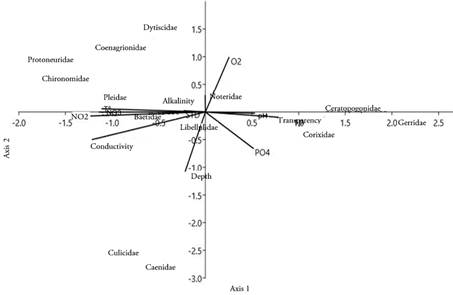
Figure 3 Canonical Correspondence Analysis (CCA) showing the relationship between the aquatic insect taxa of the studied wetlands and the abiotic variables analyzed.
The association shown between the aquatic insect community and the physicochemical conditions of the aquatic ecosystem has been highlighted in research such as that of Rocha-Ramírez et al. (2007) and Rúa-García (2015); which ratifies the close correlation between organisms and environmental factors. In this sense, physicochemical factors are considered by various authors, such as Domínguez (2009), as the aspects that have the most influence on the distribution, abundance, and richness of aquatic insects. For Salles & Ferreira-Júnior (2014), physicochemical variations along with other characteristics, such as the substrate, are determining factors that influence the adaptation of organisms. Thus, some aquatic insect groups show morphological adaptations in the shape and structure of their gills, in response to changes in water temperature and oxygen availability (Barbour et al. 1999), changes in pH can influence physiology and development of some insects, with morphological variations in response to the water acidity (Allan, 2004), and turbidity can affect eye morphology and sensory structures (Merritt et al. 2008).