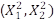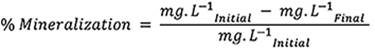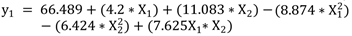INTRODUCTION
The current demand for food has increased the need for greater agricultural production worldwide, which causes the exponential use of pesticides (Sun et al. 2018), which are considered toxic substances for pest control and to increase crop yields (Vagi & Petsas, 2019) and generate a significant impact on the environment if their means of propagation are not controlled (Badii & Landeros, 2015). The Department of Valle del Cauca in Colombia is a particular case of the above inference and is the area of influence of this research study, so it is necessary to develop research on technologies for the treatment of wastewater containing pesticides in the region.
Wastewater containing pesticides is generated by the uncontrolled washing activity of spraying equipment and is mainly discharged into water sources, which is the most important means of transportation and affectation, thus creating the need to propose treatment alternatives capable of degrading substances that are not compatible with classical biological treatments, due to their high toxicity. Advanced oxidation processes (AOP) can be used as a pretreatment step to reduce high toxic concentrations to the point where inhibition is not so significant (Liberatore et al. 2012) or to obtain total mineralization of the pollutants, according to the objective of each treatment.
One of the most widely used AOPs for the decontamination of wastewater containing pesticides is photo-Fenton Photocatalysis, which consists of a mixture of hydrogen peroxide (H2O2) and divalent iron (Fe2+) catalyst species in low amounts, operating at room temperature and under acidic pH values (Vagi & Petsas, 2019), mediated in a parabolic compound collector in the presence of solar radiation. This process has proven effective in the removal of several organic compounds and pathogens (Aguas et al. 2017; Serra-Clusellas et al. 2018). However, the acidic conditions under which this treatment is effective make the process economically unattractive (De la Obra et al. 2017; Esteban García et al. 2018), subject to this, most of these AOPs pose a huge challenge concerning their operational costs, sustainability, and overall feasibility (Sgroi et al. 2020), making the development of experiments requires time, labor, and money.
As an alternative to counteract the limitations of experimentation, modeling is implemented from the general design and arises to replace the hours spent in the laboratory with fast computer simulations to obtain the correct predictions of the process behavior, taking into account an advance in this field related to sustainability and the use of technologies (Gojun et al. 2021). This points out the relevance of proposing a model for the treatment of wastewater containing pesticides by homogeneous photo-Fenton photocatalysis to enhance its advantages and reduce the limitations involved.
The modeling for the photo-Fenton process has been developed with several objectives, one of them was related to knowing the degradation kinetics through a mathematical model that describes the degradation of a dye by photolysis, which provided important information about the behavior of the system that depends on the structure of the model pollutant, the type of iron catalyst and the presence of ultraviolet light, which influences the overall efficiency of the process (Kusic et al. 2009), another similar case where a mathematical model was developed using kinetics for acetic acid consumption and first-order kinetics for hydrogen peroxide photolysis, estimating the apparent kinetic constants and testing the accuracy of the model under different experimental conditions to evidence its predictive capability (Sannino et al. 2013).
Artificial neural networks (ANN) have also been used as modeling alternatives together with the genetic algorithm for the optimization of several parameters such as H2O2 dosage, treatment time, and pH among others, allowing a prediction performance and validation of the optimal results through experimental data, confirming the reliability of this approach (Talwar et al. 2019). The response surface methodology (RSM) has also had its contribution oriented to provide a simple model capable of quantitatively describing the system performance within the experimental domain studied, through an analysis of influential variables such as inlet pH, outlet pH, the hydraulic retention time (HRT) and the H2O2 dose concerning the response variable of the percentage decolorization of a dye, resulting in a simple polynomial equation capable of predicting color removal in a wide range of experimental conditions, and also showed that the pH of the reactor and the HRT are the key factors that influenced the system response (Donadelli et al. 2020).
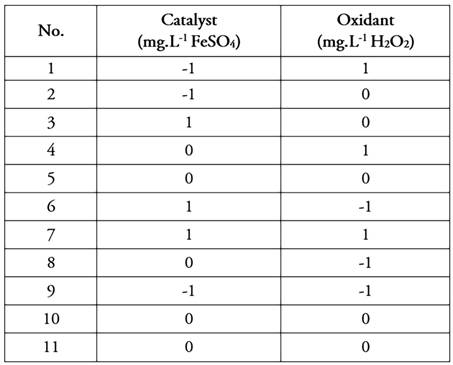
Therefore, a mathematical model is proposed for the decontamination of agro-industrial wastewater (with Carbendazim content) mediated by a homogeneous photocatalytic advanced oxidation process photo-Fenton, having as model factors in the process the H2O2 concentration at three levels (100, 1020, and 2000 mg.L-1), the FeSO4 concentration in three levels (41.55, 442.35 and 841.75 mg.L-1). The independent variable will be total organic carbon (TOC), which measures at the beginning and end of each experimental combination, considering the accumulated UV energy of 0 and 16.5 W.m-2, respectively. The combinations of experiments were randomized in the statistical program StatGraphics Centurion XIX, which yielded a total of eleven (11) experimental runs with replication at the central points of the H2O2 and FeSO4 variables.
MATERIALS AND METHODS
During the research, a methodology was developed consisting of four main phases: pesticide selection, wastewater characterization, experimental set-up, and modeling and analysis.
Pesticide selection. The selection of the pesticide is carried out through two subphases: the first one is a pre-selection according to the frequency of use in the Department of Valle del Cauca, where initially the crop zones were analyzed, classified according to the area and the pesticides of periodic use in the areas of greater extension are identified. In the second subphase, a toxicological and physicochemical classification was carried out, in addition to identifying the proportions of pesticides used in each case.
Wastewater and characterization. This phase consisted of defining the origin of the wastewater, which in this case was characterized as effluents from the washing of spraying equipment after field application of the product on the crop, determining its physicochemical characteristics, and the need for prior conditioning if necessary. In this phase, the effluent was characterized in terms of chemical oxygen demand (COD) (section 5220D. Closed reflux - Colorimetric method), biological oxygen demand (five days) BOD5 (section 5210D. Respirometric method), total suspended solids TSS (section 2540D. Total suspended solids dried at 103-105 °C), temperature (section 2550B. Laboratory and field methods), color (section 2120C. Single wavelength spectrophotometric method), turbidity (section 2130B. Nephelometric method), conductivity (section 2510 B. Laboratory method), pH, alkalinity (section 2320B. Titration method), acidity (section 2310B. Titration method), and total hardness (section 2340C. EDTA Titrimetric method), following the analytical techniques established in the Standard Methods for Water and Wastewater Analysis (Lipps et al. 2022).
Experimental set-up. Homogeneous photocatalysis is carried out in a composite parabolic collector (CPC), measured by an iron sulfate catalyst FeSO4 and the oxidizing agent hydrogen peroxide H2O2 with the incidence of solar UV radiation on the treatment system. The CPC is in an east-west direction, so that the opening plane is perpendicular to the sun's rays, thus achieving greater efficiency in radiation collection. The recirculation system consists of a 50 L.min-1 constant flow pump (the flow rate was adjusted to 42 L.min-1 through a 1.5" globe regulating valve) and a 3/4" AC type single phase electric motor (3450 RPM, 115 V, 50/60 Hz, and 1.3 Amps). The treatment volume is 20 L, the pH is constantly adjusted to 3.0 units with HCl and NaOH solutions.
Model factors in the process are the FeSO4 catalyst dose for which three levels are established: 41.55, 442.35, and 841.75 mg.L-1; the oxidizing agent H2O2 for which three levels are established: 100, 1050, and 2000 mg.L-1. The pH variable was always maintained at a fixed level of 3 units since the photo-Fenton process benefits at acid pH (López-Vinent et al. 2021; Prete et al. 2021).
The accumulated energy is evaluated at two instants: at the zero moments of the treatment, which represent 0 W.m-2, and at 16.5 W.m-2, when this last value is reached, the experimental process is terminated. An Acadus S50 radiometer with sensitivity throughout the UV range and solar energy as a source of radiation is used for its measurement.
The response variable is related to the TOC measurements at each accumulated energy value for each combination of FeSO4-H2O2 experiments. To monitor the photodegradation of the Carbendazim pesticide by TOC analysis, Shimadzu TOC-5050 analyzers are used, and samples are filtered with a 0.45 μm membrane filter. Aliquots for analysis (30 ml of solution) are taken directly from the feed tank in amber bottles to avoid photodegradation during transport to the laboratory.
The calculation of mineralization is calculated using equation 1.
Where:
% mineralization: percentage of mineralization in each combination FeSO4-H2O2.
mg.L-1 Initial: TOC measurement at the accumulated energy of 0 W.m-2 for each combination FeSO4-H2O2.
mg.L-1 Final: TOC measurement at the cumulative energy of 16 W.m-2 for each combination FeSO4-H2O2.
With the levels of the independent variables, through the statistical tool StatGraphics Centurion XIX, the different randomized combinations are elaborated.
Modeling and analysis. It is proposed to perform a multiple linear regression model taking into account a factorial design 32: two variables in three levels, as detailed in the initial methodological description. The aim is to determine the influence of the FeSO4 and H2O2 variables on each TOC response using ANOVA analysis, which allows the significance of the influential variables in the process to be determined using a p-value; then the multiple linear regression model is carried out, which results in a mathematical equation that represents the treatment process as a function of the variables studied and allows predictions to be made with an acceptable error. Subsequently, a response surface is elaborated to determine the maximum optimization routes in the process, which can determine the maximum degradation value that could be obtained under the experimental conditions evaluated. This methodological part is carried out with the statistical software RStudio Version 2023.12.1+402.
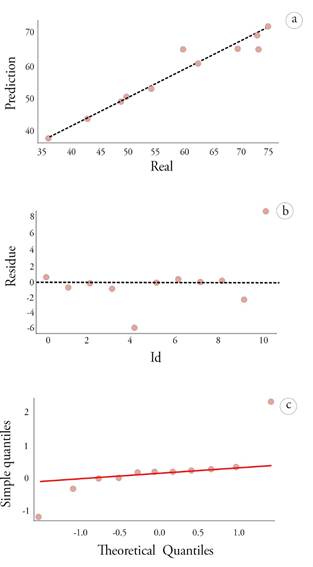
Model validation was performed using the leave-one-out cross validation (LOOCV) technique, which is ideal for small data sets. In LOOCV, each point in the data set is sequentially excluded as a test set, while the rest is used to train the model. Then, the performance of the model with the excluded point is evaluated. This process is repeated for all points in the data set, ensuring thorough validation. Additionally, the accuracy and precision of the model were evaluated using residual analysis and the ordinary least squares assumption.
The analysis and interpretation of the results are done considering the relevance of the variables studied on the model based on the significance of each one of them and the R2 value, specifying the degree of influence of each one of them on the response, in addition, to know the best optimization routes where the response variable is maximized. The correlation between the variables, the distribution of the response variable, and the model fit is analyzed in the model.
RESULTS AND DISCUSSION
The results are described taking into consideration the 4 phases proposed in the methodological design.
Pesticide selection. The pesticide selection phase resulted in the fact that the crops with the largest sowing area in the Department of Valle del Cauca are sugar cane and coffee, as well as those with the highest demand in terms of consumption of these chemical agents. Thus, 5 types were evaluated in terms of toxicity and mobility, selecting Carbendazim fungicide (in its commercial presentation with 500 g.L-1 of active ingredient) as the study pesticide because it is moderately toxic and can move in the environment.
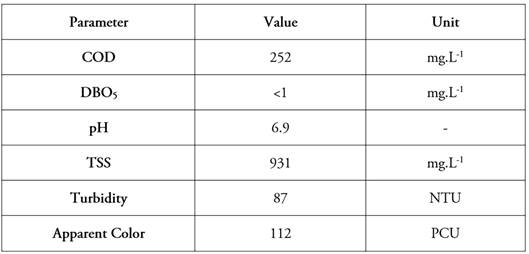
Wastewater and characterization. Two samples from the fumigation equipment washing are provided and analyzed for the characterization parameters defined in the methodological design, as detailed in table 1.
The low BOD5/COD fraction indicates an important limiting factor of low biodegradability very typical of these wastewaters (Gomes Júnior et al. 2021), which justifies the photo-Fenton process as a pretreatment step to reduce the toxic load and improve the effluent conditions. Considering the pH, it is important to stabilize it to acidic conditions since the AOP is directly affected in terms of iron photoabsorption (Gonçalves et al. 2020).
On the other hand, the values of TSS = 931 mg.L-1, Turbidity = 87 nephelometric turbidity units (NTU), and apparent color = 112 platinum cobalt units PCU, generate a limiting factor in the photo-Fenton process due to the high concentration of suspended solids that promote a light scattering effect, which is also reflected in the high color and turbidity of the real water sample (Gomes Júnior et al. 2021). The above supports the implementation of synthetic water, prepared by adding 200 ml of a 1:100 dilution of the commercial product to the total treatment volume of 20 L, which represents a working concentration of the fungicide Carbendazim of 50 mg.L-1 (approximately 100 mg.L-1 COD and 20 mg.L-1 TOC) in the treatment process.
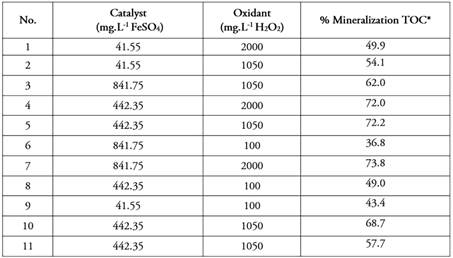
Experimental set-up. Table 2 presents the results of 11 experiments for each FeSO4-H2O2 combination with two additional replicates at the center point randomized in the statistical tool StatGraphics Centurion XIX. It should be clarified that the degradation percentages reported for TOC are at the maximum accumulated energy of 16 W.m-2.
For the programming of the model, the variables must be coded according to the following information:
FeSO4 = X1
H2O2 = X2
% Degradation TOC = Y
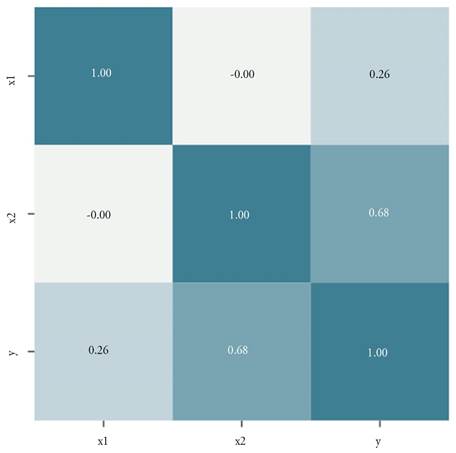
Modeling and analysis. A first analysis before starting with the development of the different models is to know the correlation between each independent variable and the associated response. Figure 1 illustrates this correlation by determining that in the case of variable Y the highest correlation is with variable X2.
With the ANOVA analysis for the response with TOC, the best fit model of the variables is given considering the interaction between FeSO4 and H2O2 (X1 * X2) and the quadratic terms of each one of them 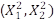 with p-values <0.05 which specifies the significance of the terms.
with p-values <0.05 which specifies the significance of the terms.
With the ANOVA information, the multiple linear regression model (MLR) is developed for which, the independent variables must be recorded according to their levels: low level = -1, medium level = 0 and high level = 1. Table 3 represents the experiments developed with the recoded variables.
The MLR again specifies the significance of the terms described in the ANOVA with the conditions of p-values<0.05 and an adjusted R2 of 0.8547, which determines the relevance of the model. Equation 2 shows the MLR model for the treatment of the synthetic water sample with 50 mg.L-1 Carbendazim in a photo-Fenton process, in a range of FeSO4 from 41.55 - 841.75 mg.L-1 and H2O2 from 100 - 2000 mg.L-1, having as response variable the percentage of mineralization in terms of TOC.
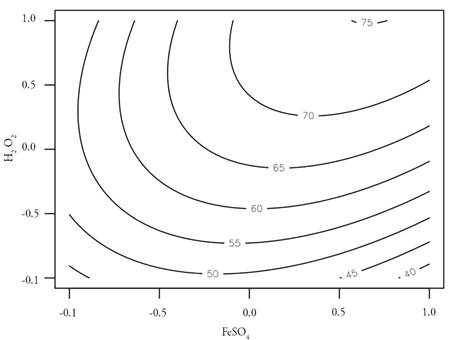
With the MLR data, the response surface is made for the experimental data, to know graphically the optimal conditions of the process and to see more clearly the behavior of the influential variables in the TOC degradation.
The response surface illustrated in figure 2 shows that the maximum degradation value occurs at the high level of both factors, i.e., for FeSO4= 841.75 mg.L-1 y H2O2= 200 mg.L-1 with 75% degradation. This result is consistent with figure 1 which showed the highest correlation between X2 (H2O2) and Y (% TOC degradation) response; this indicates that higher doses of oxidizing agent have higher degradations for this case. However, values close to this same percentage of degradation occur at the medium level of FeSO4 and high H2O2 with 72.2 % and the medium level of FeSO4 and medium H2O2 with 72.0 %. The degradation by TOC is affected as the oxidizing agent conditions decrease.
The canonical.path function allows through the MLR model and the response surface, to know the best optimization path of the experimental conditions, resulting in a maximum degradation obtained by the treatment process of 75 % and it is not possible to reach higher values even if the doses of catalyst and oxidizing agent were increased. Furthermore, the function corroborated that these maximum TOC degradations occurred at values close to the high levels of the independent variables.
In the methodology, the LOOCV technique was employed, taking advantage of the entire data to train and test the model, which reduces the bias in estimation compared to other validation techniques. According to Kassambara (2017), the estimated test error will always be constant with LOOCV over the entire data set. The average results show an RMSE of 4.537, R-squared of 0.860, and MAE of 2.869, concluding that the best model fit is the one represented in equation 2. To reinforce the validation of the model, figure 3 shows the pattern followed by the residuals, which was random, without trend or constant variance, and adjusted to a normal distribution, ratifying the ordinary least squares assumptions for the model.
Several research studies have been conducted regarding the analysis of influencing variables and the optimization of these parameters in Fenton and photo-Fenton processes for the removal of different compounds. For instance, Schenone et al. (2015) employed a photo-Fenton process to eliminate the herbicide 2,4-Dichlorophenoxyacetic acid (2,4-D) in a solar simulator. They applied a factorial experimental design combined with RSM to evaluate the influence of temperature and peroxide concentration on herbicide degradation. The results demonstrated that herbicide conversions after 180 minutes fit a quadratic model, utilizing multiple linear regression analysis to calculate model coefficients and variance analysis.
On the other hand, Mitsika et al. (2013) addressed the removal of the insecticide Acetamiprid using a Fenton process exploring different concentrations of H2O2 and Fe2+ and employing a central composite design to examine the effects of these concentrations on insecticide degradation kinetics. The results showed a satisfactory fit to a quadratic model and highlighted that an increase in hydrogen peroxide concentration accelerated insecticide degradation kinetics.
Saini & Kumar (2016) investigated the removal of the organophosphate insecticide Chlorpyrifos through a Fenton oxidation process. They used a central composite design based on RSM to study the influence of variables such as initial pH, H2O2, and catalyst concentrations on insecticide removal efficiency. The results indicated that H2O2 was a crucial factor in the reaction and that its concentration significantly affected Chlorpyrifos removal efficacy.
As a specific case, Toor et al. (2021) studied the removal of TOC and color in pig wastewater using a Fenton process, with prior biological and coagulation-flocculation pretreatments. They employed response surface methodology to optimize FeSO4 and H2O2 doses, achieving significant TOC and color removal under optimal conditions. FeSO4 was observed as the most influential factor in TOC removal, while H2O2 was crucial for color removal, emphasizing the importance of these parameters in the process.
In summary, the analyzed studies demonstrate the effectiveness of the Fenton process and its variations in removing contaminants such as herbicides, insecticides, and organic compounds in wastewater, underscoring the importance of optimizing operational conditions to achieve greater efficiency in degrading these compounds.
This research modeled the homogeneous photocatalysis process photo-Fenton for the treatment of the synthetic water sample with 50 mg.L-1 Carbendazim in a photo-Fenton process, in a range of FeSO4 from 41.55 - 841.75 mg.L-1 and H2O2 from 100 - 2000 mg.L-1, having as response variable the percentage of mineralization in terms of TOC. The fitting method implemented for the TOC response was multiple linear regression resulting in a model with an adjusted R2 = 0.8547. The model was validated using residual analysis, indicating that the errors are independent, follow a normal distribution, and have constant variance. Therefore, the model and the conclusions derived from it are valid.
The comparative analysis conducted in this study reveals a trend towards optimizing operational conditions in Fenton processes to achieve greater efficiency in degrading harmful chemical compounds present in wastewater.
There is a convergence in the use of advanced experimental designs, such as factorial experimental design and RSM, to assess the influence of key variables like H2O2 concentration and catalyst concentration (Fe2+) on the degradation kinetics of contaminants like herbicides, insecticides, and organic compounds.
The results indicate that increasing H2O2 concentration leads to faster degradation kinetics of the studied contaminants, suggesting that hydrogen peroxide plays a crucial role as a limiting reagent in Fenton oxidation processes. Furthermore, the importance of fitting to mathematical models, such as the quadratic model, to adequately describe the kinetics of contaminant elimination is evident, along with the use of central composite designs and quadratic polynomial regression analysis to establish significant relationships between operational variables and contaminant removal efficiency, thus facilitating treatment conditions optimization.
Practically speaking, it is noteworthy that optimizing operational variables like FeSO4 and H2O2 dosage can result in significant contaminant removal, as observed in TOC reduction. These findings underscore the feasibility and effectiveness of Fenton processes and their variations for treating wastewater contaminated with organic and inorganic chemical compounds.