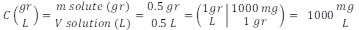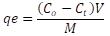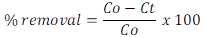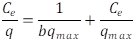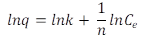INTRODUCTION
The release of effluents from the textile industry with dyes into the environment generates negative impacts, not only because it adds color to the water, affecting photosynthetic activity, but because it changes the parameters of the chemical oxygen demand (COD), biochemical oxygen demand (BOD), pH, and total solids. In addition, these discharges contain organic compounds and heavy metals that are difficult to degrade, producing an impact on the aquatic ecosystem (Islam et al. 2023).
There are around 100,000 dyes, all of them with complex chemical structures, making them resistant to fading from exposure to light, heat, water, detergents, and many chemicals (Hanafi & Sapawe, 2020). One of the most used dyes is the 3,7-bis(dimethylamino)phenazationium chloride, known generically as methylene blue (MB), which belongs to the phenothiazine family. It has the CI number 52015, and its CI name is basic blue 9. It is mainly used in the staining of cotton, wood, silk, paper, polyacrylonitrile, modified nylon, among other products (Oladoye et al. 2022). During its use, a certain amount of dye is released into bodies of water. Without proper treatment, this dye is stable and can remain in the environment for prolonged periods of time, since it is not biodegradable (Sun et al. 2019).
One of the physicochemical techniques for removing dyes is adsorption. It is a widely used method due to its simple design, ease of operation, and flexibility. The adsorption magnitude depends, largely, on the nature of the adsorbent and the molecules being adsorbed, as well as the concentration and temperature (Hashem et al. 2020). One of the best and most used adsorbents in the treatment of textile effluents is activated carbon. However, its production and regeneration are expensive, so economical and efficient adsorbent materials have been sought (Luo et al 2020).
In this regard, most Latin American governments provide financial support for the development of projects focusing on the handling and utilization of organic waste. Therefore, there is a wide range of agricultural and agro-industrial residues that have been explored. The most studied residues are fruit peels and seeds, and sugar cane bagasse, among others, which have demonstrated to have a high removal capacity for a series of dyes of industrial interest (Meili et al. 2019). Grass waste is also considered organic (Cadavid-Rodríguez & Bolaños-Valencia, 2015); however, it has been undervalued due to its high moisture content and low calorific power.
Camalote grass (Paspalum fasciculatum Willd) is a perennial grass with high fiber content and little protein (4.44%), so it does not meet the protein requirements of adult cattle grazing. This species is classified as a weed because it grows spontaneously and is even considered a very aggressive species that displaces other forage grasses of better nutritional quality (Chacón & Gliessman, 1982). Camalote grass is abundant in tropical areas, and its final destination is burning or accumulating in large quantities in open dumps.
Therefore, the interest of this work is to use Camalote grass as an efficient and low-cost adsorbent for the removal of methylene blue (MB) from aqueous media, under controlled conditions of concentration, contact time, and pH of the solution.
MATERIALS AND METHODS
Camalote grass was collected within the facilities of the Universidad Popular de la Chontalpa, in the municipality of Cárdenas, Tabasco, Mexico, whose geographical coordinates are N 17°57'36’’ and W 93°21'55’’. Methylene blue was acquired from Sigma -Aldrich. HCl and NaOH are J.T. Baker brand.
Adsorbent Preparation. The grass was cut at ground level and dried for two days in the sun. Subsequently, it was passed through an electric mill to reduce its size. The particle size of the adsorbent was selected using a steel sieve with a mesh #60.
Point of Zero Charge (PZC). 50 ml of distilled water were added to five 100 ml Erlenmeyer flasks, adjusting the pH in each solution to a range of 3 to 7 using 0.1 M HCl and NaOH solutions. 0.5 g of dry Camalote grass were added to these solutions and were stirred constantly at 225 rpm for 48 hours at room temperature. At the end of this process, the final pH value was measured. pH readings for the solutions were made using a Hanna® HI98103 pH meter.
Methylene blue solutions preparation. A stock solution of the cationic methylene blue (MB) dye was prepared at a mass concentration of 0.5 g/0.5 L, obtaining a concentration of 1,000 mg L-1 (Equation 1).
Volumetric dilutions were made from this solution to obtain solutions at concentrations of 20, 40, 60, 80, 100 mg L-1.
Calibration curve of the MB solution. A Thermo Scientific Evolution 300 UV-Visible spectrophotometer was used for a complete wavelength scan from 200 to 800 nm. The maximum adsorption wavelength was found at 664 nm, which was taken as a reference to create a calibration curve with MB solutions at concentrations of 20, 40, 60, 80, and 100 mgL-1. The absorbance readings were processed using the OriginPro8 program, performing a linear fitting by the Y= mx+b least squares method, resulting in a straight line equation, which was used to calculate the concentration present in an unknown sample.
Adsorption Test
pH Effect. 100 mg of Camalote grass was added to 50 ml of each of the 20, 40, 60, 80, 100 mg L-1 solutions of the MB dye. The pH was adjusted on a scale of 4-8 using 0.1 M HCl and NaOH solutions. The solutions were stirred at room temperature at 225 rpm for 6 h. The absorbance of the solutions was measured using a UV-Vis spectrophotometer. Contact time: 100 mg of Camalote grass was added to 50 ml of each of the 20, 40, 60, 80, 100 mgL-1 solutions of the MB dye. They were stirred at room temperature, at 225 rpm, pH 8. Aliquots were taken at intervals of 5, 10, 20, 30, 40, 50, 60, 120, 180, 240, 300, 360, and 1440 min. The absorbance of the solutions was measured using a UV-Vis spectrophotometer. Removal percentage: The amount of dye adsorbed over the measured amount of adsorbent and the percentage of color removed was calculated using equations 2 and 3.
Where C0= initial concentration of MB (mg/L); Ct= MB concentration at some time t; V= volume of solution (L) and M= mass of adsorbent (g).
Determination of functional groups. To acquire data by FTIR, a small amount of sample is taken and put on the ATR crystal. Spectra were acquired using a Nicolet iS5 FTIR spectrophotometer. Thermo Scientific: ZeS glass; acquisition range 4,000-400 cm-1; number of scans 64, resolution 4 and gain 4.
Study of equilibrium systems
Langmuir Isotherm. Experimental data were used to calculate isotherms using equation 4:
Where Ce (mg L-1) is the concentration of dye solution at equilibrium, and q (mg g-1) is the mass of adsorbed dye per gram of adsorbent. qmax is the dye mass that 1 g of adsorbent can adsorb when the monolayer is complete, and b is the isotherm constant for a particular adsorbate-adsorbent combination (parameter reflecting the affinity of the adsorbent for the adsorbate). qmax and b are calculated from the slopes (1/qmax) and intercepts (1/b qmax) of the linear lines of Ce/q.
Freundlich Isotherms. The isotherms were calculated using the linearized equation 5.
Where q is the amount of solute removed per unit mass of adsorbent (mg g-1), Ce is the concentration of MB at equilibrium (mg dm-3), K is the equilibrium constant (mgg-1 (dm3 mg-1)-1/n), and n is a constant related to the affinity between the adsorbent and the solute.
RESULTS AND DISCUSSION
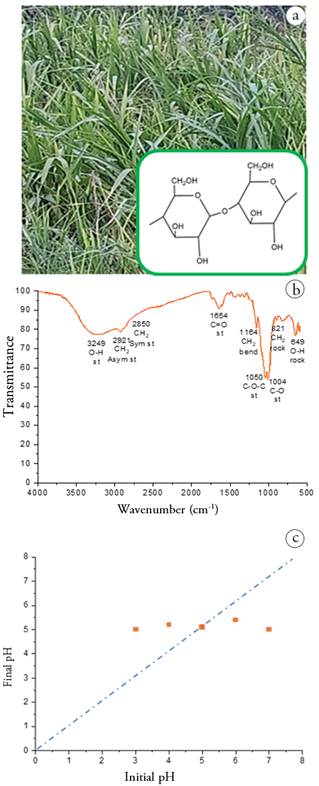
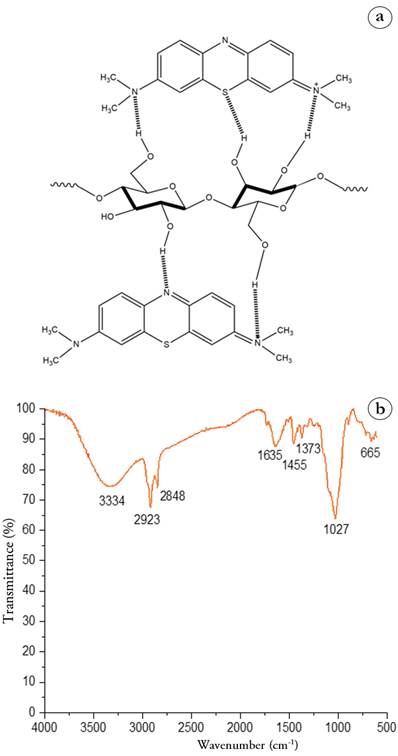
Adsorbent Characterization. The identification of the functional groups of the Camalote grass (Figure 1a) was carried out using the infrared spectroscopy technique. Figure 1b shows the FTIR spectrum where the peaks for the O-H bond are observed at 3,249 cm-1 stretching and 649 cm-1 torsion; for the C-H bond of the methylene group CH2 at 2,921 cm-1 asymmetric stretching, 2,850 cm-1 symmetric stretching, and 821 cm-1 torsion; the peak at 1,654 cm-1 corresponds to a C=O bond of the lignin structure; the peak at 1,164 cm-1 corresponds to the H2C-O-H bond; the peak at 1,050 cm-1 is assigned to the C-O-C β-glycosidic bond; and the peak at 1,004 cm-1 corresponds to the C-O bonds.
Likewise, it was considered important to determine the Point of Zero Charge value, which is one of the most important factors in the adsorption process, since it allows establishing the optimal pH to obtain the highest percentage of adsorbate removal. Figure 1c shows the graph of the initial pH vs final pH. The pHpzc was obtained by adjusting the line to the origin. The change in slope suggested the pHpzc value = 5.05. Therefore, at a pH greater than 5.05 the surface of the adsorbent will be negatively charged and will favor the adsorption of MB given its cationic structure (Rios et al. 2020).
Effect of pH on the methylene blue removal. Figure 2 shows the effect of pH of the initial solution on the amount of dye adsorbed in the concentration range of 20-100 mgL-1 MB. Observations indicate that the amount of dye adsorbed increases as the pH of the solution becomes more basic, this is associated with the pHpzc value = 5.05; values above the surface are found to negatively absorb the cationic dye more efficiently. Thus, the highest values for qe (mgg-1) and % removal for each of the concentrations at a pH 8 were obtained. The effect was best observed at the concentration of 100 mgL-1, since the amount of adsorbed dye was increased from 19 mgg-1 to 42.5 mgg-1 and from 38% to 85.5% with an increase in pH from 4 to 8.
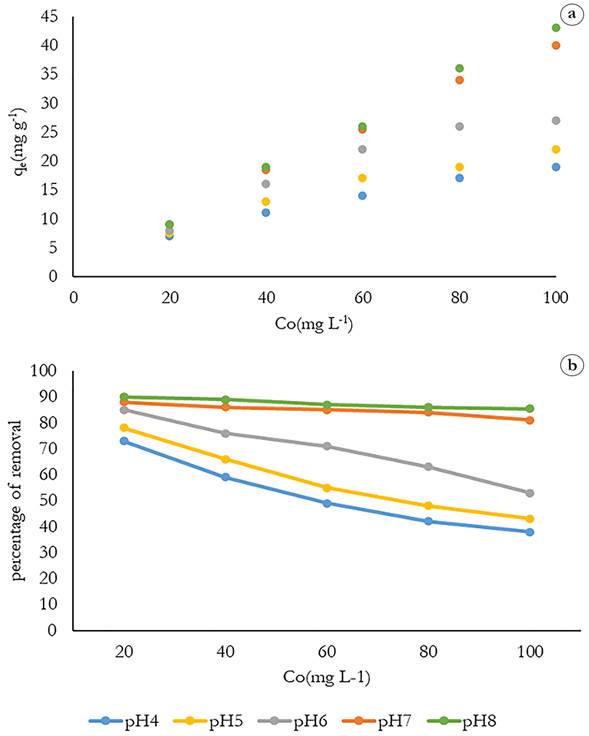
Figure 2 Effect of pH on the methylene blue removal on: a) the removal capacity; b) the removal percentage.
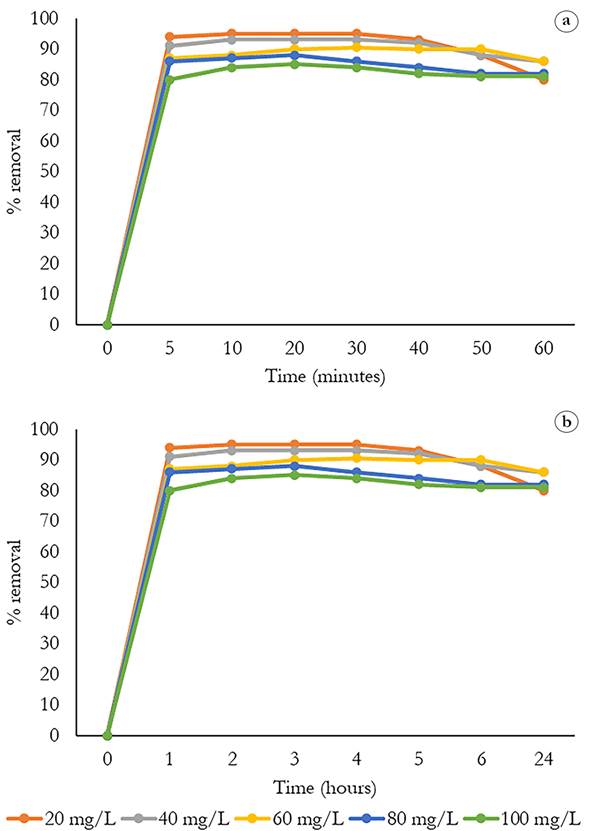
Determination of contact time. Figure 3a shows the removal percentages from 0 to 60 minutes for each of the study concentrations, where it is observed that after 5 min for the concentrations of 20 and 40 mgL-1 a removal percentage greater than 80 is achieved, while for concentrations of 60, 80, and 100 mgL-1 it is close to 80 percent. Subsequently, there is a tendency towards small increases in the value of the removal percentage in a period of 60 min, with the concentration of 20 mgL-1 reaching a percentage close to 100%. The contact time was left until completing 24 h, where it was observed that the removal percentage values remained constant (Figure 3b). The contact time established to remove the MB is 30 minutes, during which time the maximum removal percentage is reached. After this time the values remain constant.
Studies of MB-adsorbent equilibrium systems.
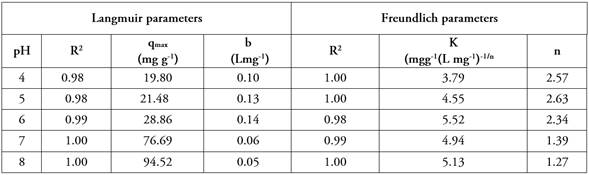
Table 1 shows the values of the Langmuir and Freundlich isotherm constants obtained from the linearity of the experimental data obtained by UV-Visible spectroscopy after adsorption of MB on the Camalote grass. The qmax values for the Langmuir isotherm range from 19.80 to 94.52 mg g-1 and b from 0.10 to 0.05 Lmg-1; while for the Freundlich isotherm, K values ranging from 3.79 to 5.13 (mgg-1 (L mg-1)-1/n and n from 2.6 to 1.27 are obtained. These values are higher than those reported by Hameed (2009) in the study of grass residues as MB adsorbents, where the reported qmax is in the range from 11.31 to 58.20 mg g-1.
Also, adsorption kinetics were performed, and experimental data were correlated to determine the equilibrium systems between MB/adsorbent using the Langmuir and Freundlich models.
Figure 4 shows that the experimental data obtained fit better to Langmuir and Freundlich models at pH higher than pHPZC = 5.05. In the Langmuir model fitting at a pH of 8, qmax = 94.52 represents the maximum adsorption capacity of the solid phase, and b = 0.05 is a parameter reflecting the affinity of the adsorbent for the adsorbate. The values were comparable to those reported in the methylene blue adsorption with cotton peel (Gossypium hirsutum L.) (Zaval-Flores et al. 2024). In the Freundlich model fitting, at a 7 and 8 pH, n values close to 1 were obtained, which means that chemisorption is favored in the active sites. Similarly, K values range from 3 to 5 suggesting that methylene blue is retained by the adsorbent through chemisorption (Rivas et al. 2014).
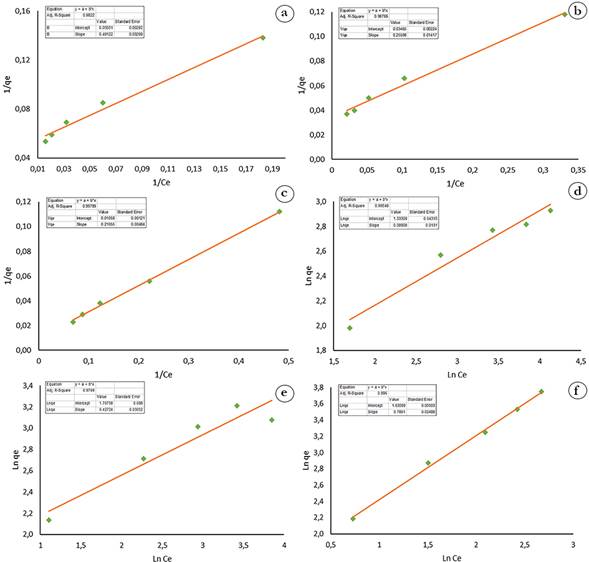
Figure 4 Fitting of the experimental data to the Langmuir and Freundlich isotherms. a) Langmuir isotherm pH 4; b) Langmuir isotherm pH 6; c) Langmuir isotherm pH 8; d) Freundlich isotherm pH 4; e) Freundlich isotherm pH 6; f) Freundlich isotherm pH 8.
Chemisorption can occur due to Camalote grass and methylene blue contain functional groups capable of interacting with each other through hydrogen bonds. Figure 5a shows a representation of the interaction between the adsorbent and methylene blue through hydrogen bonds. Figure 5b shows the FTIR spectrum obtained after the adsorption process where it is observed that the initial peaks presented in Figure 1b are shifted to longer wavelengths due to the interaction by hydrogen bonds between the links. Vibrations after the adsorption process for the O-H bond are at 3,334 cm-1 stretching and 665 cm-1 torsion; for CH2-O-H bond at 2,923 cm-1 asymmetric stretching, 2,848 cm-1 symmetrical stretching; 1,455 cm-1 bending; and C-O-C β-glycosidic bond shifted to 1,027 cm-1. A new peak appears in the spectrum at 1,455 cm-1 corresponding to the C-N bond. Results showed that methylene blue was adsorbed within the Camalote grass structure (Jiang & Hu, 2019).
In this work, it was found that for all MB dye concentrations studied, removal percentages greater than 80% occurred. The adsorption of methylene blue is pH-dependent. The maximum adsorption capacity was 94.52 mg g-1 at pH 8. The results obtained are fitted to the Freundlich and Langmuir isotherms, which shows the formation of a monolayer and a multilayer, and it is suggested that the interaction between blue methylene and the adsorbent are given by chemisorption through hydrogen bonds interactions.
Therefore, it can be concluded that Camalote grass (Paspalum fasciculatum) can be considered as an adsorbent material in the treatment of water contaminated with dyes, thus providing a solution to the accumulation of waste aimed at poor final disposal.