Introduction
Invasive aspergillosis (IA) continues to be an important cause of morbidity and mortality, difficult to manage in severely immunocompromised patients, however, with its appearance in a heterogeneous group of patients (e.g., critical ICU patients, patients with human immunodeficiency virus [HIV/ AIDS], etc.), who were not classically considered high risk, as well as more chronic forms of aspergillosis, (including those with a propensity for invasion), which have been better defined, different antifungal treatment modalities have been established for Aspergillus-associated infections according to the specific patient's condition1-11,14-58,67-293. The understanding of the different risk factors for the development of IFI/IA, which are constantly evolving, and which include, among others, the underlying malignancy, the associated condition and treatment, the presence of comorbidities, environmental exposure, and the presence of certain genetic polymorphisms in the patient, allows for a more precise risk stratification4,6,21,67-69,115,118,153, which together with the use of diagnostic algorithms, would allow the characterization of patients who would benefit from the different early intervention strategies, and the optimization of management proto-cols4,8,153,21,67,68,70,118-121. The evaluation of the clinical manifestations of the patient is an essential step, as it involves the site of infection, the severity and dynamic nature of immunosuppression, and the characteristics of the etiological agent involved, which with the use of imaging modalities (with an increasingly important role in diagnosis), and of novel and accessible diagnostic tools, useful for the detection and follow-up of the disease, allow the early recognition of the infection, the selection of an early antifungal treatment, the use of more effective antifungal drugs and the development of local clinical practice guidelines4,67.
The last few decades have seen important changes in fungal epidemiology, with a better understanding of the incidence and global and local epidemiology, which has gone from an almost uniform lack of management options, to a disease that is diagnosed more quickly and treated more aggressively with increasingly safe antifungal drugs4,8,11,21,67,68,70-75,81-84. The initiation of active prophylaxis against filamentous fungi should be considered in patients at high risk of developing invasive disease, however, in a patient with a lower risk, it is only advisable to implement close monitoring protocols, and the initiation of immediate treatment upon evidence of active infection4,21,67,68,70-75,77,81-84,91,92,95. The optimization of antifungal treatment is fundamental, including (a) selecting the optimal antifungal drug and its early initiation to improve survival rates, (b) ensuring adequate drug exposure, (c) managing drugdrug interactions, (d) maintaining adequate treatment time, and (e) implementing objective parameters for the evaluation of results115,118. Likewise, it has been established that the successful management of proven/probable IFI/IA depends not only on antifungal treatment, but also on the reduction of immunosuppression, whenever feasible, and the consideration in those patients with severe and/or refractory disease, of the initiation of additional therapies such as immunomodulation (such as granulocyte transfusions and complementary use of interferon gamma [IFN-y]) and surgery4,21,67,70.
Finally, when maximizing diagnostic accuracy, it should be considered that different treatment approaches may be necessary for a varied group of patients, as therapeutic practices may differ between hospital centers, according to local epidemiology, diagnostic tools available and/or accessible in a timely manner, and patient characteristics118,119. The implementation of antifungal stewardship (AFS) programmes allows optimizing the outcome of a patient with IFI/IA, with selection of the drugs, the dosage, the route of administration and the adequate duration of treatment, while limiting the consequences of its use, such as the emergence of antifungal resistance, adverse drug reactions and hospital costs118,121. A multifaceted strategy is needed, the first step of which is to build a multidisciplinary team with the necessary expertise, focusing on: (a) surveillance of fungal infection, and the study of new cases and/or outbreaks, (b) quality measurement of antifungal drug prescribing, and (c) improving the acceptance of diagnostic testing and the use of therapeutic drug monitoring (TDM) of antifungal agents118-121.
A detailed description of the background, methods and potential conflicts of interest can be found in the Section 1 of the guideline "Colombian Consensus on the Diagnosis and Follow-Up of Invasive Aspergillosis and Aspergillus Disease in Adult and Pediatric Patients". Summarized below are the recommendations for the prophylaxis, treatment and prevention of invasive aspergillosis. To assess the quality of the evidence and the strength of the recommendations, the modified GRADE methodology12,13 was used. It assigns each recommendation with separate ratings for the underlying quality of the evidence supporting the recommendation, and for the strength with which the recommendation is made, establishing the following levels of evidence: LOW (III): results may definitely change over time; MODERATE (II): results may change over time, but will not change dramatically; HIGH (I): the likelihood that the results will change is minimal. The strength of the recommendation (STRONG OR WEAK) was evaluated taking into account the balance between benefits and risks, quality of evidence, patient values and preferences, and cost or resource utilization Table 1 59-66.
Table 1 Scale for measuring the quality of evidence and strength of recommendations.
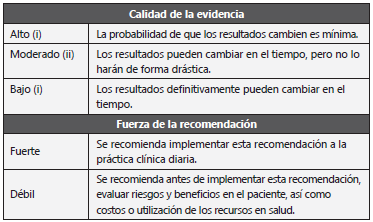
Adapted from: Andrews JC et al. 12
Recommendation
1.The consensus considers that in patients older than 13 years, with high suspicion and/or very high risk of developing an IFI/IA, either because of their baseline disease and/or local/geographic, and/or healthcare facility-related conditions that are clearly recognized, the initiation of primary antifungal prophylaxis is recommended. (strong recommendation, highquality evidence)Tables 2 -4 4,11,21,67-70.
Table 2 Risk factors and patients at risk for an IA.
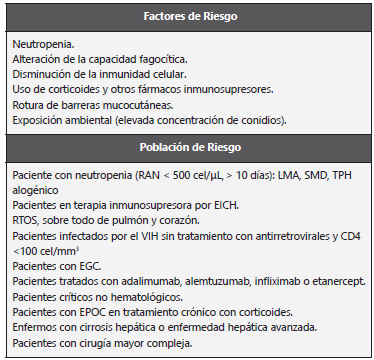
AI: Aspergilosis invasora; RAN: Recuento de neutrófilos absolutos; LMA: Leucemia mieloide aguda; SMD: Síndrome mielodisplásico; TPH: Trasplante de progenitores hematopoyéticos; EICH: Enfermedad injerto contra hospedero; RTOS: Receptores de trasplante de órganos sólidos; VIH: Virus de inmunodeficiencia humana; Enfermedad granulomatosa crónica; EPOC: Enfermedad pulmonar obstructiva crónica. Adapted from: Pemán J et al. 33.
Table 3 Risk of IA in SOTR patients.
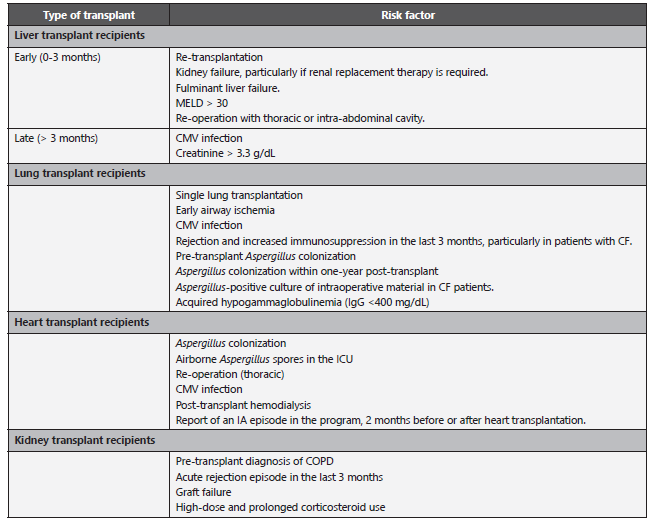
CF: Cystic Fibrosis; CMV: Cytomegalovirus; COPD: Chronic obstructive pulmonary disease; IA: invasive aspergillosis; ICU: Intensive Care Unit; MELD: Model for End-stage Liver Disease; SOTR: Solid Organ Transplant Recipient. Adapted from: Husain S et al. 8.
Table 4 Incidence of IA in pediatric population.
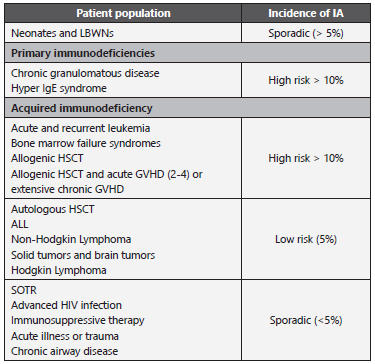
ALL: Acute Lymphoblastic Leukemia; GVHD: graft-versus-host disease; HIV: human immunodeficiency virus; HSCT: hematopoietic stem cell transplantation; IA: invasive aspergillosis; SOTR: Solid Organ Transplant. Adapted from: Groll AH et al. 50; Tragiannidis A et al. 51; García-Vidal C et al. 67
Table 5 Aspergillus species and antifungal susceptibility.
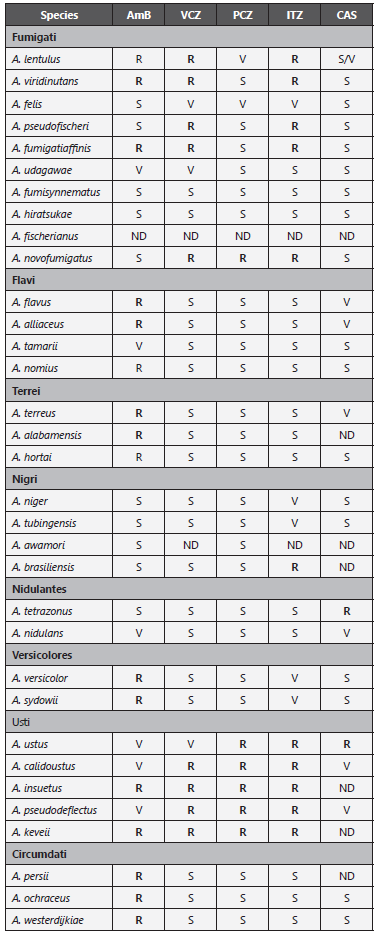
+For practical reasons, for PCZ, MIC: ≥0.25 mg/L is considered resistant; for AmB, ITZ and VCZ, MIC ≥ 2 mg/L is considered resistant.
AmB: Amphotericin B; VCZ: Voriconazole; PCZ: Posaconazole; ITZ: Itraconazole; CAS: Caspofungin; S: Susceptible; R: Resistant; V: Variable; ND: No data.
Adapted from: Samson RA et al. (52); Gautier M et al. 56
2. The consensus recommends the initiation of primary antifungal prophylaxis, universal and/or directed against filamentous fungi, in patients older than 13 years, with high suspicion of developing an IFI/IA. The populations that according to their specific clinical condition are recognized for the initiation of primary antifungal prophylaxis are: (a) hematological patients with leukemia with profound and prolonged neutropenia, (b) HSCT patients during neutropenic phase, (c) patients HSCT recipients in GVHD phase, moderate to severe stage, and (d) patients in whom intensified immunosuppression isrequired. (strong recommendation, highquality evidence)8,14,22,36,68,70-84.
3. It is recommended in patients SOTR (solid organ transplant recipients), with high suspicion of developing an IFI/IA, the initiation of primary universal and/or directed antifungal prophylaxis against filamentous fungi. The populations that according to their specific clinical condition, are recognized for the initiation of primary antifungal prophylaxis are: (a) lung transplant recipients, (b) heart-lung transplant recipients, (c) pancreas transplant recipients, and (d) high-risk liver transplant recipients in need of dialytic support during transplantation. (strong recommendation, high-quality evidence)Table 8 4,8,21,67,70,76.
Recommendation
4. The consensus does not recommend the initiation of universal primary antifungal prophylaxis against IFI caused by filamentous fungi in patients hospitalized in the ICU. (strong recommendation, moderatequality evidence)4,21,31,67,70,85.
5. The consensus recommends in the ICU hospitalized patient, the initiation of primary antifungal prophylaxis directed against IFI caused by filamentous fungi, with the following conditions: (a) SOTR, with an increased risk of microenvironmental exposure, (b) COPD, (c) high-dose corticosteroid therapy, (d) acute liver failure, (e) burns, (f) severe bacterial infection, or (e) malnutrition. (strong recommendation, moderatequality evidence)4,21,31,67,70,85.
Recommendation
6. The consensus does not recommend the initiation of universal primary antifungal prophylaxis against IFI caused by filamentous fungi in HIV/AIDS patients. The decision to initiate targeted primary antifungal prophylaxis in patients with CD4 count <100 cells/mm 3 should be made on an individual basis. (weak recommendation, low-quality evidence)86,87.
Recommendation
7. The consensus recommends that in the patient with a diagnosis of ALL, the decision to initiate primary antifungal prophylaxis directed against IFI caused by filamentous fungi should be made on an individualized basis, according to the institutional prevalence of IFI caused by Aspergillus spp. and the risk of chemotherapeutic treatment in the induction phase. Consideration should be given to evaluating possible drug-drug interactions. (strong recommendation, high-quality evidence) (Annexes 1 and 2) 4,21,67,68,70,72-75.
Recommendation
8. It is recommended in the patient with a hematologic malignancy and/or HSCT recipients, in whom the development of profound and prolonged neutropenia (absolute neutrophil count [ANC]: <500 cells/pL, >7days) is expected, the initiation of primary, universal and/or targeted antifungal prophylaxis against IFI caused by filamentous fungi , with the following conditions: (a) AML or MDS, in induction phase, (b) allogeneic HSCT, until neutrophil recovery, (c) allogeneic HSCT, in GVHD phase, moderate or severe stage, requiring corticosteroid therapy (prednisone [PDN] >1 mg/kg/day), or other immunosuppressive therapy. (strong recommendation, high-quality evidence)Table 2 4,21,81-84,67,68,70-75.
9. The consensus does not recommend the initiation of primary, universal and/or targeted antifungal prophylaxis against IFI caused by filamentous fungi in the patient undergoing autologous HSCT. (strong recommendation, moderate-quality evidence)4,21,67,68,70.
Recommendation
10. The consensus recommends in the HSCT patient with chronic immunosuppression associated with GVHD, on corticosteroid therapy, (PDN equivalent > 1mg/kg/d, for > 2 weeks) together with other anti-GVHD therapies, (TNF-a antagonist drugs [infliximab, adalimumab, etanercept] and/or anti-lymphocyte biologic agents [rituximab, alemtuzumab]), the initiation of primary antifungal prophylaxis against IFI caused by filamentous fungi. (strong recommendation, high-quality evidence)4,6,21,75,81-84,88-92,67,68,70-74.
Recommendation
11. The consensus does not recommend in the patient undergoing biologic and/or cell therapy with TNF-a antagonist drugs ([infliximab, adalimumab, etanercept) and/or with anti-lymphocyte biologic agents (rituximab, alemtuzumab, basiliximab, daclizumab), the initiation of universal primary antifungal prophylaxis against IFI caused by filamentous fungi. (strong recommendation, moderatequality evidence)4,21,67,70.
Recommendation
12. The consensus recommends that in the SOTR patient, the decision to initiate universal primary antifungal prophylaxis against IFI caused by filamentous fungi should be made on an individualized basis and according to the institutional prevalence. Consideration should be given to evaluating possible drug-drug interactions. (strong recommendation, high-quality evidence)Table 8 4,8,21,67,70,76,93.
Recommendation
13. It is recommended in the lung transplant recipient patient (during the first post-transplant year), the initiation of primary antifungal prophylaxis, universal and/or directed against IFI caused by filamentous fungi, depending on the availability and/or timely access to diagnostic approach tools. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-Up of IA/Aspergillus Disease) Tables 7 and 8, Annex 3) 4,8,21,67,70,76,78,94-97.
Table 6 Diseases caused by Aspergillus spp.
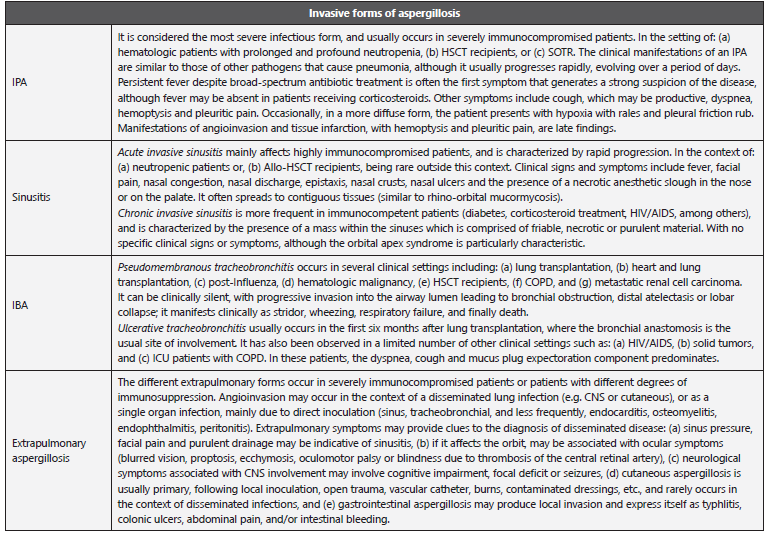
IA: invasive aspergillosis; IPA: Invasive pulmonary aspergillosis; IBA: Invasive bronchial aspergillosis; HSCT: Hematopoietic stem-cell transplantation; SOTR: Solid organ transplant recipient; HIV: Human Immunodeficiency Virus; COPD: Chronic obstructive pulmonary disease.; ICU: Intensive Care Unit.
Adapted from: Gregg KS et al. 11; Hope WW et al. 58; García-Vidal C et al. 67.
Table 7 Pathological and imaging findings in diseases caused by Aspergillus spp.
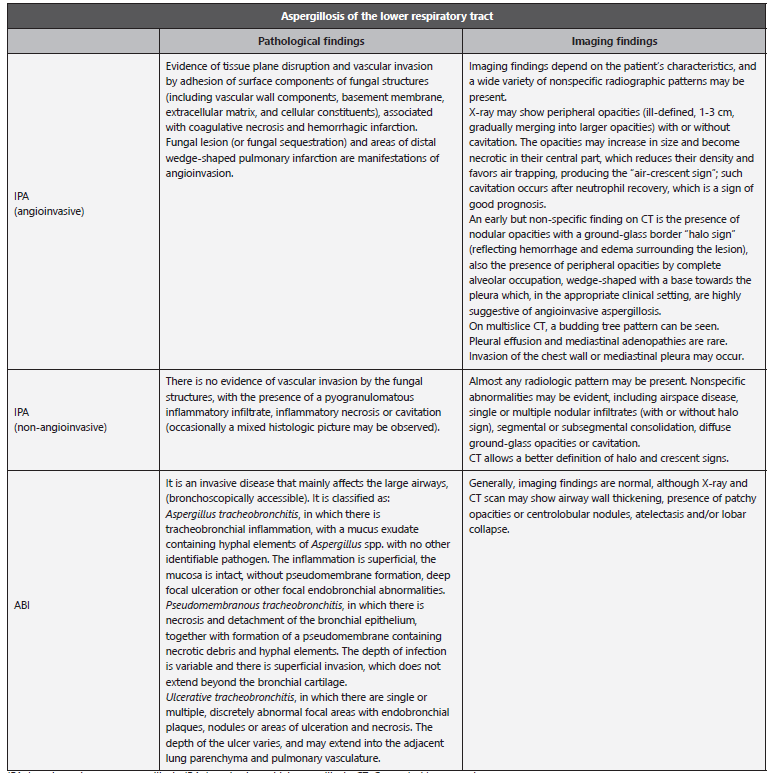
IPA: Invasive pulmonary aspergillosis; IBA: Invasive bronchial aspergillosis; CT: Computed tomography.
Adapted from: Gregg KS et al. 11; Hope WW et al. 58; Orlowski HL et al. 129; Hage CA et al. 461; Chong S et al. 467; Murthy JM et al. 468.
Table 8 Antifungal prophylaxis in SOTR.
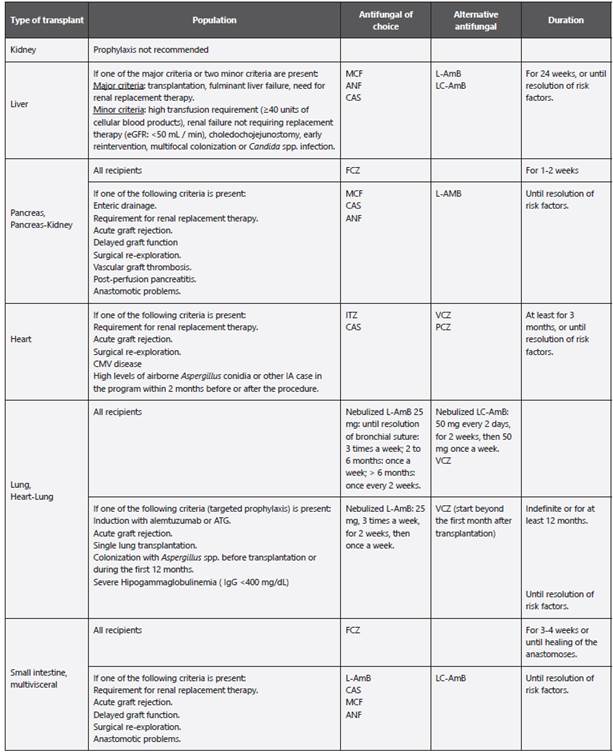
LC-AmB: Amphotericin B lipid complex; L-AmB: Liposomal amphotericin B; CAS: Caspofungin; ANF: Anidulafungin; MCF: Micafungin; FCZ: Fluconazole; ITZ: Itraconazole; ATG: Anti-thymocyte globulin; eGFR: Estimated glomerular filtration rate; IgG: Immunoglobulin G; IgG: Immunoglobulin G; SOT: Solid organ transplantation; SOTR: Solid organ transplant recipient; CMV: Cytomegalovirus. Adapted from: Husain S et al. 8; Ullmann AJ et al. 21; García-Vidal C et al. 67.
14. The consensus recommends in the lung transplant recipient patient, (during the first year post-transplant), the initiation of primary antifungal prophylaxis directed against IFI caused by filamentous fungi, with at least one of the following conditions present: (a) demonstrated fungal colonization, from pre-transplant intra-operative culture and/or within the first year post-transplant, (b) early and prolonged airway ischemia at the site of anastomosis, (c) administration of daclizumab, thymoglobulin or alemtuzumab, and/or treatment with high-dose corticosteroids, (d) CMV infection, (e) acute and chronic repeated rejection, or (f) acquired hypogammaglobulinemia (IgG <400 mg/dl). (strong recommendation, moderatequality evidence)Table 8 4,8,21,67,70,76,78,95-97.
Recommendation
15. The consensus does not recommend the initiation of universal primary antifungal prophylaxis against IFI caused by filamentous fungi in heart transplant recipients. The decision to initiate targeted antifungal prophylaxis should be made on an individual basis, taking into account the risk of QTc prolongation due to the use of azoles. (strong recommendation, high-quality evidence) (Annexes 1and 2) 4,8,21,67,70,97-100
16. It is recommended in the heart transplant recipient patient the initiation of primary antifungal prophylaxis directed against IFI caused by filamentous fungi, with at least one of the following conditions present: (a) patient with thoracic re-operation, (b) demonstrated fungal colonization, from intra-opeative culture, with no imaging abnormalities present, (c) CMV infection, (d) post-transplant he-modialysis, (e) hospitalized in ICU, and demonstration of the presence of conidia of Aspergillus spp. in the environment, (f) administration of sirolimus or tacrolimus, (g) acquired hypogammaglobulinemia (IgG <400mg/dl), or (h) report of an episode of IA in any patient, within the cardiac transplant program, 2 months before or after the cardiac transplantation. (strong recommendation, high-quality evidence)Table 8 4,8,21,67,70,97-99.
17. The consensus does not recommend the initiation of universal primary antifungal prophylaxis against IFI caused by filamentous fungi in liver transplant recipients. The decision to initiate antifungal prophylaxis directed against filamentous fungi should be made on an individual basis. (strong recommendation, high-quality evidence)Table 8 4,8,21,36,67,70,100-103.
18. Initiation of primary antifungal prophylaxis directed against filamentous fungal IFI is recommended in the liver transplant recipient patient with at least one of the following conditions present: (a) liver retransplantation, (b) post-transplant hemodialysis, (c) renal replacement therapy at the time, or within 7 days post-transplant, (d) fulminant hepatic failure, (e) MELD scale > 30 points, (f) ICU admission or requirement for corticosteroid treatment, four weeks prior to transplantation, (g) transfusion of > 15 U of packed red blood cells during transplant surgery, (h) surgical re-intervention involving the thoracic and/ or intra-abdominal cavity, or (i) choledochojejunostomy. (strong recommendation, highquality evidence) (table .8)4,8,21,36,67,70,100,101.
19. The consensus does not recommend the initiation of universal primary antifungal prophylaxis against IFI caused by filamentous fungi in kidney transplant recipients. The decision to initiate antifungal prophylaxis directed against filamentous fungi should be made on an individual basis. (strong recommendation, low-quality evidence)4,8,21,67,70.
20. Initiation of primary antifungal prophylaxis directed against IFI caused by filamentous fungi is recommended in the kidney transplant recipient patient with at least one of the following conditions present: (a) pre-trans-plant COPD, (b) delayed graft function, (c) post-transplant bloodstream infection, or (d) acute graft rejection. (strong recommendation, low-quality evidence)Table 8 4,8,21,67,70.
Recommendation
21. The consensus does not recommend the initiation of universal primary antifungal prophylaxis against IFI caused by filamentous fungi in the patient with a diagnosis of COPD. It is recommended to initiate targeted primary antifungal prophylaxis with at least one of the following conditions present: (a) treatment with high-dose systemic and cumulative corticosteroids, (b) refractory antibiotic treatment, or (c) ICU admission. (strong recommendation, moderate-quality evidence)4,21,67,70,104.
22. The consensus does not recommend the initiation of universal primary antifungal prophylaxis against IFI caused by filamentous fungi in patients with liver failure. The decision to initiate antifungal prophylaxis directed against filamentous fungi should be made on an individual basis. (strong recommendation, moderate-quality evidence)4,21,67,70.
23. The consensus does not recommend the initiation of universal primary antifungal prophylaxis against IFI caused by filamentous fungi in the severely burned patient. The decision to initiate antifungal prophylaxis directed against filamentous fungi should be made on an individual basis, with at least one of the following conditions present: (a) high percentage of total body surface area involved with burn injuries, (b) prolonged length of hospital stay. (strong recommendation, moderatequalityevidence)4,21,67,70,105.
Recommendation
24. The consensus recommends the use of antifungal drugs to initiate primary, universal and/or targeted antifungal prophylaxis in high-risk patients to reduce the incidence of IFI/ IA. The drugs of choice are the azoles (posaconazole [PCZ], voriconazole [VCZ], itraconazole [ITZ]: [standard dose]) orally [PO.] or intravenously [IV]. Although ITZ is considered to be effective, its use may be limited by its absorption and tolerability. (strong recommendation, highqualityevidence)Table 9, Annexes 1 and 2) 4,21,67,70.
Table 9 Systemic antifungal agents for treatment of IA. ADME, Doses.
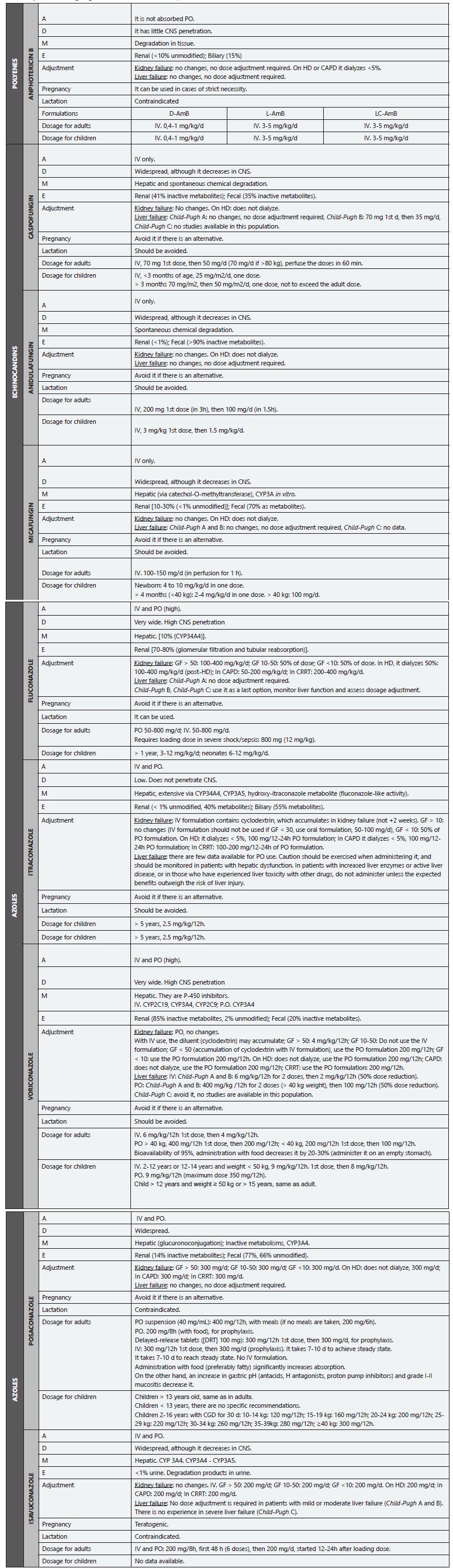
IA: invasive aspergillosis; A: Administration; D: Distribution; M: Metabolism; E: Excretion; D-AmB: Amphotericin B deoxycholate; L-AmB: Liposomal amphotericin B; LC-AmB: Amphotericin B lipid complex; GF: Glomerular filtration; IV: Intravenous route; PO: Oral route; d: Day/days; h: Hour/hours; g: Grams; mg: Milligrams; kg: Kilograms; HD: Hemodialysis; CAPD: Continuous Ambulatory Peritoneal Dialysis; CRRT: Continuous Renal Replacement Therapy; CGD: Chronic Granulomatous Disease; CNS: Central Nervous System.
Adapted from: Cuenca-Estrella M. 124; Mensa-Pueyo J et al. 469; Gilbert D et al. 470; Jenks JD. et al. 471; Ghannoum MA y Perfect JR (eds) 472; Ruiz-Camps I et al. 473; Bellmann R et al.474; Lewis RE. 475; Nett JE et al. 476; Welzen MEB et al. 477.
25. The consensus considers that the new ITZ formulation (ITZ-SUBA, capsules, 65 mg / 12h, with meals) PO., is an alternative to initiate primary, universal and / or directed antifungal prophylaxis against IFI caused by filamentous fungi. However, the drug is not available in many countries. (strong recommendation, high-quality evidence)21,70,106.
26. In patients with prolonged primary antifungal prophylaxis with an azole (VCZ, PCZ or ITZ), and/or together with the administration of a drug with pharmacological interaction with azoles, it is recommended that therapeutic monitoring of antifungal drugs (TDM) be performed to improve antifungal efficacy, evaluate therapeutic failure and reduce pharmacological toxicity. (strong recommendation, high-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Tables 9 and 10, Annexes 4 and 5) 4,21,67,70.
Table 10 Recommendations for TDM.
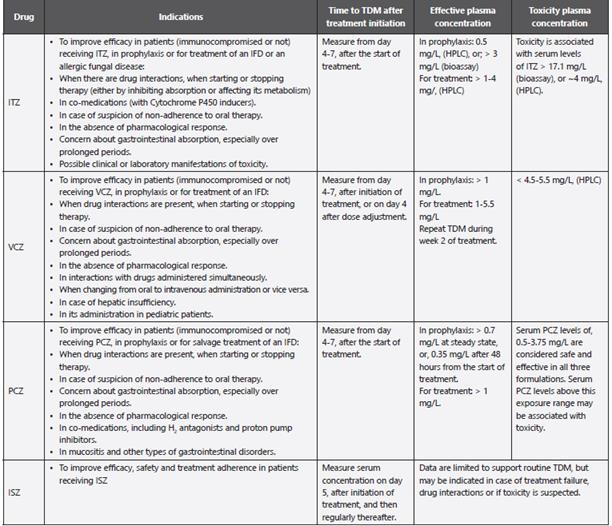
TDM: Therapeutic drug monitoring of antifungal agents; ITZ: Itraconazole; VCZ: Voriconazole; PCZ: Posaconazole; ISZ: Isavuconazole; IFD: Invasive fungal disease; HPLC: High-performance liquid chromatography.
Adapted from: Fortún J et al. 20; Ullmann AJ et al. 21; Ashbee HR et al. 151; Cendejas-Bueno E. et al. 478.
27. The consensus does not consider the use of isavucona-zole (ISZ) as an alternative to initiate primary, universal and/or targeted antifungal prophylaxis against IFI caused by filamentous fungi. (strong recommendation, high-quality evidence)21,70,107.
28. The consensus recommends that in the high-risk patient with a diagnosis of hematologic malignancy and/ or HSCT, with/without profound and prolonged neutropenia, avoid coadministration of an azole drug with other potentially toxic drugs (e.g., vinca alkaloids and others). Consider evaluating potential drug-drug interactions when choosing an antifungal drug for primary, universal and/or targeted antifungal prophylaxis against IFI caused by filamentous fungi. (strong recommendation, highquality evidence) (Annexes 1 and 2) 21,70,81.
29. The lipid formulations of AmB (liposomal AmB [L-AmB] or lipid complex AmB [LC-AmB]) can be considered as an alternative for primary, universal and/or targeted anti-fungal prophylaxis against IFI caused by filamentous fungi in the high-risk patient when azole use is contraindi-cated and/or not tolerated. The use of AmB deoxycholate (D-AmB) should be reserved for resource-limited settings without access to alternative antifungal drugs. (strong recommendation, highquality evidence)Table 9,Annexes 1 and 2) 4,21,67,70,108-111.
30. Nebulized AmB formulations are an alternative for primary, universal and/or targeted antifungal prophylaxis against IFI caused by filamentous fungi in the high-risk patient with hematologic malignancy or HSCT or lung transplant recipient. (weak recommendation, lowquality evidence)4,21,67,70,108-111.
31. An echinocandin (caspofungin [CAS] or micafungin [MCF], standard dose), is an alternative for primary, universal and/ or targeted antifungal prophylaxis against IFI caused by filamentous fungi, when the use of azoles or polyenes is contra-indicated and/or not tolerated. (strong recommendation, high-quality evidence) (Annexes 1 and 2) 4,21,67,70.
a. In the patient at risk of developing an IFI/IA, what is the recommendation for choosing the drug type, dose, and duration of primary anti-fungal prophylaxis, according to the populationat risk?
Patient with hematologic malignancy and/or HSCT
32. The consensus considers that in high-risk patients with hematologic malignancy and/or HSCT, with/without profound and prolonged neutropenia, PCZ PO. or VCZ PO. are the drugs of choice for initiating universal and/or targeted primary antifungal prophylaxis against IFI/IA. The decision to initiate primary targeted antifungal prophylaxis should be made on an individual basis. (strong recommendation, high-quality evidence)Table 9 4,21,67,70,72,73,77,112-114.
33. The consensus recommends in the high-risk patient with a diagnosis of hematologic malignancy (AML/MDS, in induction), with/without profound and prolonged neu-tropenia, the initiation of primary antifungal prophylaxis with: (a) PCZ (delayed-release tablets, TLR [300 mg/12h, two doses, then 300 mg/d, with meals], or suspension [200 mg/8h], with carbonated beverages), or (b) VCZ (PO., 200 mg/12h), or (c) ITZ (200 mg/12h), or (d) L-AmB (nebulized, 12.5 mg, X2/wk + fluconazole [FCZ] PO.), or (e) an echinocandin (IV., CAS [50 mg/d], MCF [50-100 mg/d]). (strong recommendation, high-quality evidence)Table 9 4,21,67,68,70-75,81-84.
34. It is considered that in the high-risk patient with a diagnosis of hematological malignancy (AML/MDS, in induction), with profound and prolonged neutropenia, the duration of primary antifungal prophylaxis will depend on the resolution of the neutropenia. (strong recommendation, high-quality evidence)4,21,67,68,70 - 75,81-84.
35. It is recommended in the high-risk allogeneic RTPH patient, in neutropenic phase, the initiation of primary antifungal prophylaxis with: (a) PCZ (TLR [300 mg/12h, two doses, then 300 mg/d, with meals], or suspension [200 mg/8h], with carbonated drinks), or (b) VCZ (PO., 200 mg/12h), or (c) L-AmB (nebulized, 12.5 mg, X2/wk + FCZ PO., or (d) an echinocandin (IV., CAS [50 mg/d], MCF [50100 mg/d]). (strong recommendation, high-quality evidence) 4,21,67,68,70-75,81-84.
36. It is considered that in the high-risk allogeneic RTPH patient, in neutropenic phase, the duration of primary antifungal prophylaxis will be up to day +75/100. (strong recommendation, high-quality evidence)4,21,67,68,70-75,81-84.
37. It is recommended in the allogeneic HSCT patient, in moderate to severe GVHD phase and/or intense im-munosuppression, the initiation of primary antifungal prophylaxis with: (a) PCZ (tablets [300 mg/12h, two doses, then 300 mg/d, with meals], or suspension [200 mg/8h], with carbonated beverages), or (b) VCZ (PO., 200 mg/12h), or (c) L-AmB (nebulized, 12.5 mg, X2/wk + FCZ PO.), or (d) an echinocandin (IV., CAS [50 mg/d], MCF [50100 mg/d]). (strong recommendation, highquality evidence)4,21,67,68,70-75,81-84
38 In the allogeneic HSCT patient with moderate to severe GVHD phase and/or intense immunosuppression, the duration of primary antifungal prophylaxis is considered to depend on the resolution of the GVHD and/or for the duration of immunosuppression. (strong recommendation, high-quality evidence)4,6,21,67,68,70-75,8-84,88-92.
SOTR patient
39. The consensus considers that in the high-risk SOTR patient, PCZ PO., or VCZ PO., or nebulized L-AmB, are the drugs of choice to initiate primary antifungal prophylaxis, universal and/or directed against IFI/IA. The decision to initiate primary antifungal prophylaxis should be made on an individual basis and it is recommended to monitor liver function to evaluate toxicity related to the use of azoles. (strong recommendation, highquality evidence)Table 9 4,8,21,67,70,76.
40. The consensus recommends in the high-risk lung transplant recipient patient, the initiation of primary antifun-gal prophylaxis with: (a) PCZ (tablets, 300 mg/d), or (b) VCZ (PO., 200 mg/12h, X3-6/month), or (c) ITZ (PO., 200 mg/12h, X3-6/month), or (d) D-AmB (nebulized, 25 mg/d, for 4 d, then 25 mg/wk, X7/wk), or (e) L-AmB (nebulized, 50 mg/d, for 4 d, then 50 mg/wk, X 7/wk), or (e) LC-AmB(nebulized, 50 mg/48h, X2/wk, then 50 mg/wk, X13wk), or (f) an echinocandin (IV., CAS [50 mg/d], MCF [50-100 mg/d], X3-4/month). In the single lung transplant recipient patient, initiation of primary antifungal prophylaxis systemically is considered. (strong recommendation, moderate-quality evidence)4,8,21,67,70,76,78,95-97.
41. The consensus considers that in the high-risk lung transplant recipient patient the duration of universal and/or targeted primary antifungal prophylaxis should be indefinite or a minimum of 12 months. The duration will depend on airway inspection, the results of surveillance respiratory cultures, and the patient's risk factors. (strong recommendation, moderate-quality evidence)Table 8 4,8,21,67,70,76,78,95-97.
42. The consensus recommends in the high-risk heart transplant recipient patient the initiation of primary antifungal prophylaxis with: (a) PCZ (tablets [300 mg/12h, two doses, then 300 mg/d, with meals], or suspension [200 mg/8h], with carbonated beverages), or (b) VCZ (PO., 200 mg/12h, X50-150/d) or (c) ITZ (PO.,200 mg/12h, X50- 150/d), or (d) an echinocandin (IV., CAS [50 mg/d], MCF [50-100 mg/d], X120/d). (strong recommendation, high-quality evidence)4,8,21,67,70,97-99.
43. The consensus considers that in the high-risk heart transplant recipient patient, the duration of universal and/or targeted primary antifungal prophylaxis should be indefinite or a minimum of 120 days. (strong recommendation, high-quality evidence)Table 8 4,8,21,67,70,97-99.
44. The consensus recommends in the high-risk liver transplant recipient patient the initiation of primary antifun-gal prophylaxis with: (a) VCZ (PO., 200 mg/12h), or (b) LAmB (IV., 3-5 mg/d), or (c) an echinocandin (IV., CAS [70 mg, day 1, then 50 mg/d], ANF [200 mg, day 1, then 100
3. In the patient at risk of developing an IFI/IA, in which clinical situations is the initiation of secondary antifungal prophylaxis recommended?
Recommendation
46. The consensus considers that in the immunocompromised patient, the initiation of secondary antifungal prophylaxis prevents relapse of an IFI/IA associated with a previous episode. The populations that according to their specific clinical condition, are recognized for the initiation of secondary antifungal prophylaxis are: (a) allogeneic HSCT in early phase with profound and prolonged neutropenia, (b) allogeneic HSCT in chronic, acute or extensive GVHD phase, (c) patients undergoing T-cell depletion therapy and/or with high doses of corticosteroids. The initiation of secondary antifungal prophylaxis should always be based on the response to previous antifungal therapy. (strong recommendation, moderate-quality evidence) (Annex 6) 4,21,67,71,83.
47. It is recommended in the patient with a diagnosis of a previous proven/probable IFI/IA, who is going to undergo an allogeneic HSCT, or with a new risk period, and who presents unresectable foci of Aspergillus disease, to reduce the risk of recurrence, the initiation of secondary antifungal prophylaxis with an active drug against IFI caused by filamentous fungi. (strong recommendation, moderate-quality evidence)4,21,67,71,83.
48. It is recommended in the patient with a diagnosis of a previous proven/probable IA/IFI, who will undergo allo-geneic HSCT, or with a new risk period, and who presents resectable foci of an Aspergillus disease, the consideration of surgical debridement, along with initiation of secondary antifungal prophylaxis with an active drug against IFI caused by filamentous fungi. (strong recommendation, moderate-quality evidence)Table 1 1). 4,21,67,71,83.
a. In the patient at risk of developing an IFI/IA, what is the recommendation for choosing the drug type, dose and duration of secondary prophylaxis, according to the population at risk?
Recommendation
49. It is recommended in patients with a diagnosis of a previous proven/probable IA/IFI, who will undergo allogeneic HSCT, or with new risk period, the initiation of secondary antifungal prophylaxis with: (a) VCZ (PO., 200 mg/12h), or (b) CAS (IV., 70 mg, one dose, then, 50 mg/d, [if body weight is <80 kg]), until the graft is stable, followed by ITZ (PO., 400 mg/12h), or (c) L-AmB (IV., 3-5 mg/d), followed by VCZ (PO., 200 mg/12h). (strong recommendation, moderate-quality evidence) (Annex 6) 4,21,67,71,83.
SUB SECTION II:
EMPIRICAL ANTIFUNGAL TREATMENT (EAFT) AND/OR DIAGNOSTIC-DRIVEN ANTIFUNGAL TREATMENT (DAFT) OF IFI/IA
1. In the patient at risk of developing an IFI/IA, is the initiation of an EAFT and/or a DAFT recommended? In which clinical situations is the initiation of an EAFT and/or a DAFT recommended?
Empiric antifungal treatment
50. In order to decrease the incidence and/or related mortality in the hospitalized patient older than 13 years, with high suspicion of developing an IFI/IA where an incidence of IA >10% is established, the consensus recommends early initiation of an EAFT against filamentous fungi, while a complete diagnostic evaluation is performed. (strong recommendation, high-quality evidence) (Annex 3) 4,21,67,115,116.
51. In the hematologic and/or HSCT patient with profound neutropenia for a prolonged period (>10 days) and/ or persistent fever (>96 hours) despite adequate use of broad-spectrum antibiotic therapy (AbAE), and without availability and/or timely access to diagnostic tools, the consensus recommends the initiation of an EAFT against filamentous fungi. (strong recommendation, high-quality evidence)4,21,67,115,116.
52. The consensus does not recommend in the hematologic patient with neutropenia of short duration (<10 days) the initiation of an EAFT against filamentous fungi, unless other diagnostic findings and/or tests demonstrate proven/probable IFA/IA. (strong recommendation, high-quality evidence)4,67.
53. The consensus does not recommend in the immunocompromised patient with ongoing primary antifungal prophylaxis the initiation of an EAFT against filamentous fungi. (strong recommendation, high-quality evidence)4,21,67,115.
Diagnostic-driven antifungal treatment
54. The consensus considers, in high-risk patients receiving primary antifungal prophylaxis (VCZ or PCZ), who develop persistent fever or other clinical manifestations that raise suspicion of the development of an IFI/IA, to consider a breakthrough invasive infection. It is recommended to perform TDM within a DAFT approach to improve antifungal efficacy, evaluate therapeutic failure and decrease the drug toxicity. (strong recommendation, moderate-quality evidence) (I Diagnosis and Followup of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Table 10, Annexes4 and 5) 4,21,67,115.
a. In the patient at risk of developing an IFI/IA, what is the standard of action according to the clinical scenario?
Recommendation
Empiric antifungal treatment
55. The consensus recommends in the high-risk immuno-compromised patient, regardless of the presence of fever, the initiation of an EAFT against filamentous fungi, with at least one of the following conditions present: (a) history of previous proven/probable IFI/IA, (b) neutropenic patient with fungal colonization, (c) presence of characteristic clinical symptoms (pleuritic chest pain, blood-tinged sputum and/or hemoptysis), or (d) presence of suggestive clinical signs (new-onset pneumonia, tenderness, or edema around the sinuses or orbital area, ulcerative lesions or eschar in the nasal area). (strong recommendation, moderate qualityevidence)4,21,67,115,116.
56. The consensus recommends initiation of an EAFT against filamentous fungi, in the high-risk hematologic patient, with at least one of the following conditions present: (a) clinical malaise or instability, with/without prior antifun-gal prophylaxis, or (b) persistent fever refractory to antibiotic treatment, with/without neutropenia and without prior antifungal prophylaxis (or prior prophylaxis with FCZ or ITZ), and without availability or timely access to Aspergillus galactomannan antigen [AGA] and/or fungal DNA detection [PCR] results; or (c) fever refractory to antibiotic treatment, with/without neutropenia, and/or clinical signs/symptoms of an IFI/IA and prior antifungal prophylaxis with VCZ or PCZ, and without availability or timely access to TDM results. (strong recommendation, moderate-quality evidence)4,21,115.
57. The consensus recommends initiation of an EAFT against filamentous fungi in the high-risk non-neutropenic patient, with at least one of the following conditions present: (a) persistent fever refractory to antibiotic treatment, and/ or clinical deterioration, (b) clinical symptoms characteristic of invasive disease, despite adequate use of AbAE, and without availability or timely access to diagnostic tools. (strong recommendation, moderate-quality evidence)4,21,67,115.
58. The consensus recommends in the high-risk hematolo-gic patient, with high suspicion of developing an IFI/IA, to perform periodically: (a) blood and/or microbiological cultures (urine, sputum, fecal material, and other sites [clinically indicated]), (b) multi-slice CT (sinuses, abdomen, and other sites [clinically indicated]), (c) measurement of AGA and/or PCR from serum and/or BAL, in the patient without prior antifungal prophylaxis (or prior prophylaxis with FCZ or ITZ), (d) fibrobronchoscopy (FBC) with BAL sampling and lung biopsy, (if any imaging abnormality is detected), (e) biopsy from involved site and other sitesSection 2. Colombian consensus for prophylaxis, treatment and prevention of invasive aspergillosis in adult and pediatric patients [clinically indicated], and (f) perform TDM of azole drugs. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-Up of IA/Aspergillus Disease) Tables 7 and 10, Annex 3) 4,21,67,115,117.
59. The consensus recommends in the high-risk hematological patient taking an EAFT, with a diagnosis of a proven/probable IFI/IA, to continue the initial antifungal treatment or to change the drug and/or dose, according to the identified etiological agent and its sensitivity profile by in vitro an-tifungal susceptibility testing (AFST). (strong recommendation, moderate-quality evidence)4,21,67,115.
60. The consensus recommends in the high-risk hematologic patient taking an EAFT, in whom proven/probable IFI/ IA has been ruled out, to de-escalate primary antifungal prophylaxis or discontinue the EAFT. (strong recommendation, moderate-quality evidence)4,21,67,115.
Diagnostic-driven antifungal treatment
61. The consensus considers that in the high-risk hematologic patient, a surveillance strategy guided by diagnostic tools which are available and/or accessible in a timely manner can be used. The consensus does not recommend in the patient on primary antifungal prophylaxis with VCZ or PCZ to use a DAFT strategy against a proven/ probable IFI/IA. (strong recommendation, moderate-quality evidence)4,21,67,115.
62. The consensus recommends initiation of a DAFT in high-risk hematologic patients who cannot receive primary antifungal prophylaxis against filamentous fungi. It is considered that there is no evidence for the initiation of a DAFT against proven/probable IFI/IA in other high-risk populations (such as SOTR patients). (strong recommendation, moderate-quality evidence)4,21,67,115.
63. The consensus recommends in the high-risk immuno-compromised asymptomatic patient, with/without fever, the use of fungal biomarkers (AGA, (1,3)-ß-D-glucan [BDG], and/or PCR) and/or imaging studies, as a DAFT strategy, avoiding the initiation of unnecessary treatment. It is considered that a DAFT can increase the number of documented cases of proven/probable IFI/IA, without compromising survival, being an alternative to the EAFT. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [value of Ags and/or biomarkers tests] [imaging approach for the diagnosis of IPA]) Tables 6 and 7, Annex 3) 4,67,118-121.
64. As a surveillance strategy guided by diagnostic tools in the patient with hematological malignancy and/or HSCT at high risk of an IFI/IA, the consensus considers to perform a chest multi-slice CT scan when: (a) a first positive result of AGA and/or PCR, continuing the measurement of biomarkers pending imaging results, (b) a negative result of AGA and/or PCR but persistent fever and refractory to antibiotic treatment, (c) clinical signs/symptoms of a proven/probable IFI/IA. (strong recommendation, moderate-quality evidence)4,21,67,115.
65. The consensus recommends in the patient with hemato-logic malignancy and/or HSCT at high risk of an IFI/IA, the initiation of a DAFT when: (a) 2 consecutive positive AGA and/or PCR results, or (b) > 2 intermittently positive AGA and/or PCR results within a 2-week period, or (c) a single AGA and/or PCR result and any lesion detected on the chest multi-slice CT, or (d) a characteristic lesion detected on the chest multi-slice CT. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [value of Ags and/or biomarkers tests] [imaging approach for the diagnosis of IPA]) Tables 6 and 7, Annex 3) 4,21,67,115.
66. The consensus does not recommend the initiation of a DAFT in patients with hematologic malignancy and/or HSCT at high risk of an IFI/IA, and it is considered to look for another type of associated infection when: (a) a single AGA and/or PCR result is positive, or (b) an uncharacteristic lesion detected on the chest multi-slice CT, or (c) all the results of the diagnostic tests performed are negative. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [value of Ags and/or biomarkers tests] [imaging approach for the diagnosis of IPA]) Tables 6 and 7, Annex 3) 4,67,118-121.
67. The consensus considers that in the patient at high risk of developing an IFI/IA, TDM is a complementary tool within a DAFT approach. (strong recommendation, moderate-quality evidence)Table 10, Annexes 4 and 5) 4,67,115,117.
68. As a surveillance strategy guided by diagnostic tools with availability and/or timely access to TDM results but not AGA and/or PCR in the patient with hematologic malignancy and/or HSCT, with/without neutropenia, who is clinically well/stable on primary antifungal prophylaxis (with VCZ, PCZ or L-AmB) and/or refractory fever and/or clinical signs/symptoms of proven/probable IFI/IA, the consensus recommends maintaining antifungal prophylaxis with the same drug and starting dose. (strong recommendation, moderate-quality evidence)4,21,67,115,117.
69. In the patient with hematologic malignancy and/or HSCT, with/without neutropenia, who is clinically well/ stable on primary antifungal prophylaxis (with VCZ, PCZ, or L-AmB) and/or refractory fever and/or clinical signs/ symptoms of proven/probable IFI/IA, with availability and/or timely access to TDM results but not AGA and/ or PCR, the consensus recommends to perform periodically: (a) TDM, and/or (b) blood and/or microbiological culture collection (urine, sputum, fecal material, and other sites [clinically indicated]), (c) multi-slice CT in all patients, (sinuses, abdomen, and other sites [clinically indicated]), and/or (d) FBC with BAL collection and lung biopsy. (strong recommendation, moderate qualityevidence)4,21,67,115,117.
70. In the patient with hematologic malignancy and/or HSCT, with/without neutropenia, clinically well/stable on primary antifungal prophylaxis (with VCZ, PCZ or L-AmB) and/ or refractory fever and/or clinical signs/symptoms of an IFI/IA, and a TDM result within therapeutic range and nodiagnosis of invasive disease, the consensus recommends: (a) maintain antifungal prophylaxis with the same drug and starting dose, and (b) repeat TDM at regular intervals and ensure that it remains in the therapeutic range. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Table 10, Annexes 4 and 5) 4,21,67,115,117.
71. In the patient with hematologic malignancy and/or HSCT, with/without neutropenia, clinically well/stable on primary antifungal prophylaxis (with VCZ, PCZ or L-AmB), and/or refractory fever and/or clinical signs/symptoms of an IFI/IA and a TDM result within the sub-therapeutic range and no diagnosis of invasive disease, the consensus recommends: (a) increase the dose of antifungal drug on prophylaxis, if feasible, and/or implement measures to maximize pharmacologic exposure, (b) repeat TDM at regular intervals and ensure that it remains in therapeutic range, (c) if therapeutic range is not achieved, consider changing the antifungal drug. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Table 10, Annexes 4 and 5) 4,21,67,115,117.
72. In the patient with hematologic malignancy and/or HSCT, with/without neutropenia, clinically well/stable, with primary antifungal prophylaxis (with VCZ, PCZ or L-AmB), and/or refractory fever and/or clinical signs/symptoms of an IFI/IA with diagnosis of invasive disease, independent of TDM outcome, the consensus recommends antifungal drug switching, and drug choice is determined by: (a) identified etiological agent, (b) sensitivity profile by AFST, (c) degree of immunosuppression, (d) ability to absorb the drug PO., and (e) potential to achieve therapeutic levels of the new drug. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/ Aspergillus disease]) Table 10, Annexes 4 and 5) 4,21,67,115.
73. In the high-risk patient on primary antifungal prophylaxis (with VCZ, PCZ or L-AmB), with a diagnosis of a proven/probable breakthrough IFI/IA, the consensus considers that the therapeutic approach should be performed on an individualized basis, based on: (a) the patient immunosuppression, (b) underlying disease, (c) site of infection, (d) antifungal dosing, (e) outcome of TDM, (f) change from PO. to IV. antifungal therapy, and (g) change in antifungal drug family. (strong recommendation, moderate-quality evidence)4,21,67,115.
2. In the patient at risk of developing an IFI/IA, what are the recommended EAFT and/or DAFT regimens?
Recommendation
74. The consensus recommends in the patient at high risk of developing an IFI/IA, with persistent fever and refractory to antibiotic treatment, to consider initiating an EAFT and/or a DAFT and decrease the incidence and/or mortality from an IFI/IA. (strong recommendation, highquality evidence)Table 9, Annexes 1 and 2) 4,122-126.
75. The consensus considers that in the high-risk immunocompromised patient, the decision on initiation and choice of antifungal drug within an EAFT or DAFT approach should be made on an individualized basis, according to: (a) the risk level of IFI/IA, (b) the characteristics and severity of the clinical picture, (c) the antifungal prophylaxis received, and (d) the results of biomarkers (AGA and/or CRP) and multi-slice CT (chest and/or paranasal sinuses). (strong recommendation, moderate-quality evidence)4,8,21,67,119,127.
76. The consensus considers that in the high-risk patient with hematologic malignancy and/or HSCT, with/without profound and prolonged neutropenia, the choice of an antifungal drug for an EAFT and/or a DAFT will depend on the result of the AGA and the type of prophylaxis administered according to the clinical context: (a) if the AGA is negative or not available and the patient has received prophylaxis with an extended-spectrum azole ([ESA], VCZ or PCZ) or an echinocandin (MCF), initiation of an anti-fungal treatment with L-AmB, (b) if the AGA is negative or not available and the patient has not received prophylaxis with an ESA or an echinocandin, initiation of a treatment with L-AmB, an echinocandin or VCZ, (c) if the AGA is positive and the patient has received prophylaxis with an ESA or MCF, if the prophylaxis was with an ESA, initiation of treatment with L-AmB, if the prophylaxis was with MCF, initiation of treatment with VCZ or L-AmB, (d) if the AGA is positive and the patient has not received antifungal prophylaxis, initiation of antifungal treatment with VCZ or L-AmB. (strong recommendation, high-quality evidence)Table 9, Annexes 1 and 2) 4,122-126.
a. In the patient at risk of developing an IFI/IA, what is the recommendation for the choice of drug type, dose and duration of EAFT and/or DAFT, according to the at-risk population?
Recommendation
77. In the high-risk patient with hematologic malignancy and/or HSCT, with/without profound and prolonged neutropenia, with suspected probable/possible IFI/IA, the consensus recommends initiation of an EAFT and/or a DAFT with: (a) AMB-L (IV., 3-5 mg/kg/d), or (b) an echinocandin (IV., CAS [70 mg, day 1, then 50 mg/d], ANF [200 mg, day 1, then 100 mg/d], MCF [100 mg/d]). (strong recommendation, moderate-quality evidence)4,21,67.
78. VCZ (IV., [6 mg/kg/12h, day 1, then, 4 mg/kg/12h], or PO. [200-300 mg/12h or 3-4 mg/kg/12h]) or ITZ (IV., 200 mg/12h, day 1-2, then 200 mg/d.), may be considered as an alternative to an EAFT and/or a DAFT, in the high-risk patient with hematologic malignancy and/or HSCT, with/ without profound and prolonged neutropenia, with suspected probable/possible IFI/IA, in resource-limited settings or when the use of first-line drugs is contraindicated and/or not tolerated. (strong recommendation, moderate-quality evidence)Table 9, Annexes 1 and 2) 11,21,70.
79. The consensus considers that in the high-risk patient with hematologic malignancy and/or HSCT, with/without profound and prolonged neutropenia, in whom proven/probable IFI/IA is suspected, the duration of EAFT and/or DAFT will be determined by: (a) defervescence, (b) recovery from neutropenia and GVHD phase, (c) stable clinical condition, and (d) no fungal etiologic agent has been identified. The EAFT can be stopped early if no IFI/IA is diagnosed in the course of treatment, otherwise it should be continued for the required time according to the respective IFI. (strong recommendation, moderate-quality evidence) (section: targeted antifungal treatment of IA/IPA) 4,21,67,116.
80. The consensus recommends in the high-risk non-neutrope-nic patient with suspected probable/possible IFI/IA, initiation of EAFT and/or DAFT with: (a) AMB-L (IV., 3-5 mg/kg/d), or (b) VCZ (IV., [6 mg/kg/12h, day 1, then, 4 mg/kg/12h], or PO., [200-300 mg/12h or3-4 mg/kg/12h]). (strong recommendation, moderate-quality evidence)4,21,67.
81. The consensus considers that in the high-risk non-neutro-penic patient with suspected probable/possible IFI/IA, the duration of EAFT and/or DAFT will depend on the clinical response and the disappearance of clinical, microbiological and imaging evidence of invasive disease. (strong recommendation, moderate-quality evidence) (section: targeted antifungal treatment of IA/IPA) 4,21,67,116.
SUB SECTION III: TARGETED ANTIFUNGAL TREATMENT OF IA/IPA
QUESTIONS:
1. In the adult patient with IA/invasive pulmonary aspergillosis (IPA), how is the diagnostic approach performed?
Recommendation
82. The consensus recommends in the high-risk adult patient with rapidly progressive invasive disease and/or pulmonary involvement to carry out a diagnostic approach of proven/probable IA/IPA by: (a) histopathology and/ or culture positive for Aspergillus spp. from respiratory tract specimen (induced sputum, tracheal aspirates, BAL, etc.) and/or lung biopsy and/or contiguous site (e.g., paranasal sinuses), (b) positive PCR test from lung biopsy (especially in the context of tissue infarction and necrosis) and/or BAL and/or serum, (c) positive AGA test from serum (x2) and/or BAL (x1), and (d) abnormal chest CT findings (e.g., halo sign, air crescent sign, single or multiple pulmonary nodules). (strong recommendation, high-quality evidence) (I Diagnosis and Follow-Up of IA/Aspergillus Disease) Tables 6 and 7, Annex 3) 58,128-136.
COVID-19-associated pulmonary aspergillosis (CAPA)
83. The consensus considers that in the patient with severe respiratory syndrome caused by the SARS-CoV-2 virus (COVID-19), the diagnostic approach of a proven/probable/possible COVID-19-associated pulmonary aspergi-llosis (CAPA) is similar than for a proven/probable IPA. The lack of clinical validation and the poor diagnostic performance of the available diagnostic tests should be considered in the context of a patient with severe COVD-19, which may limit their clinical utility. (strong recommendation, moderate-quality evidence)41-47,49,137,138.
a. In the patient with proven/probable IA/IPA, what are the recommended antifungal treatment regimens?
Recommendation
84. The consensus recommends that in the patient diagnosed with an IA/IPA associated with cryptic and/or considered intrinsic/primary resistant Aspergillus species, the choice of drug for initiation of primary targeted antifungal therapy should be based on: (a) AFST results, (b) the site of infection, and (c) the patient characteristics. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [Aspergillus resistance to antifungal drugs]) Table 5, Annex 6) 4,21,54,67,128,139.
85. The consensus recommends in the patient with a diagnosis of proven/probable IA/IPA, as a first choice of primary targeted antifungal therapy the use of VCZ, alone or in combination, IV. (in severe disease [6 mg/kg/12h, day 1, then, 4 mg/kg/12h], and in mild/moderate disease [400 mg/12h, day 1, then 200 mg/12h]; or PO. (patients < 40 kg [half the maintenance dose]), or IV. ISZ (200 mg/8h, day 1-2, then 200 mg/d). It is recommended to perform a TDM of VCZ to improve antifungal efficacy, evaluate therapeutic failure and decrease pharmacological toxicity. (strong recommendation, high-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Tables 9 and 10, Annexes 4 and 5) 4,21,67,68,70,88.
86. L-AmB (IV., 3-5 mg/kg/d), or LC-AmB (IV., 5 mg/kg/d) are an alternative for primary targeted antifungal therapy in the patient with a diagnosis of proven/probable IA/IPA. The consensus does not recommend the use of D-AmB for primary antifungal therapy. (strong recommendation, moderate-quality evidence)Table 9, Annexes 1 and 2) 4,21,67,68,70,88.
87. An echinocandin (IV., CAS [70 mg, day 1, then 50 mg/d], ANF [200 mg, day 1, then 100 mg/d], MCF [100 mg/d]), PCZ (IV., 300 mg/12h, day 1, then 300 mg/d), or ITZ (IV., 200 mg/12h, day 1-2, then 200 mg/d), may be considered for salvage antifungal therapy (or when contraindicated and/or azoles or polyenes are not tolerated), in the patient with a diagnosis of proven/probable IA/IPA. Routine use of an echinocandin in monotherapy as primary antifungal therapy is not recommended. (strong recommendation, moderate-quality evidence)Table 9 4,21,67,68,70,88.
88. ITZ (PO., 200 mg/8h, day 1-2, then 200-400 mg/d.), can be considered as an alternative for primary antifungal targeted therapy in the patient diagnosed with IA/IPA, with mild disease, when the use of AmB and/or echino-candins is contraindicated and/or not tolerated. The TDM is recommended to improve antifungal efficacy, evaluate therapeutic failure and reduce drug toxicity. (strong recommendation, moderate-quality evidence)Table 9,Annexes 1 and 2) 4,8,21,67,68.
89. It is considered that in the patient with a diagnosis of proven/probable IA/IPA the duration of antifungal treatment should be a minimum of 6-12 weeks. The duration will depend on: (a) the degree and duration of immunosuppression, (b) the site of infection, and/or (c) evidence of improvement of invasive disease. (strong recommendation, moderate-quality evidence)4,8,21,67,68.
Patient with CAPA
90. The consensus recommends the use of VCZ (IV., 6 mg/ kg/12h, day 1, then 4 mg/kg/12h) or ISZ (IV., 200 mg/8h, day 1-2, then 200 mg/d) as the first option for primary targeted antifungal therapy in patients with a diagnosis of proven/probable/possible CAPA. It is recommended to perform a TDM of VCZ to improve antifungal efficacy, evaluate therapeutic failure and reduce pharmacological toxicity. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Tables 9 and 10, Annexes 4 and 5) 4,21,67,68,70,116,128,140-147.
91. L-AmB (IV., 3-5 mg/kg/d) is an alternative for primary targeted antifungal therapy in the patient with a diagnosis of proven/probable/possible CAPA when there is a risk of: (a) hepatotoxicity from VCZ use, (b) intolerance or allergy from azole use, (c) drug-drug interactions. (strong recommendation, moderate-quality evidence)Table 9, Annexes 1 and 2) 4,21,67,68,70,116,128,140-147.
92. It is considered that in the patient with a diagnosis of proven/ probable/possible CAPA, the duration ofantifungal treatment should be established on an individualized basis and should be a minimum of 6-12 weeks, depending on the clinical and imaging evolution of the patient. (strong recommendation, moderate-quality evidence)4,21,67,68,70,116,128,140-147.
Recommendation
93. The consensus recommends in the patient older than 13 years, with a diagnosis of proven/probable IA/IPA, the initiation of primary targeted antifungal therapy. (strong recommendation, high-quality evidence)4,21,67,68,70,88.
94. The consensus recommends in specific patient populations, according to their clinical context and risk group, the initiation of primary targeted antifungal therapy. High-risk situations for the development of IA/IPA are: (a) profound and prolonged neutropenia (RAN: <500 cells/ [ μL, > 7 days), (b) hematologic malignancy, (c) allogeneic HSCT, (d) lung transplant recipient without filamentous fungal prophylaxis. (strong recommendation, highquality evidence)Tables 2-4 148,149.
95. The consensus recommends that in the patient with a diagnosis of a proven IA/IPA the approach for initiation of primary targeted antifungal therapy based on AFST results, and with the relevant clinical isolate with intrinsic/primary resistance is: (a) AFST of Aspergillus spp, with a minimum inhibitory concentration (MIC) to VCZ > 2 mg/L, start with L-AmB in monotherapy, or VCZ + echinocandin in combination, (b) AFST of A. tubingensis (part of A. niger complex), or A. lentulus (part of A. fumigatus complex) with an MIC to VCZ = 2 mg/L, start with VCZ + echinocandin in combination or L-AmB in monotherapy, (c) isolate of A. niger complex, avoid starting with ISZ, (d) AFST of A. calidoustus (part of A. ustus complex) with an MIC at VCZ > 2 mg/L, start with L-AmB, (d) AFST of A. terreus or A. alliaceus (part of A. flavus complex), start with VCZ or ISZ (if susceptible, in vitro). (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [Aspergillus resistance to antifungal drugs]) Table 5, Annex 6) 4,21,54,67,128,134,139.
96. The consensus considers that in the high risk hospitalized patient older than 13 years of age, where an azole resistance rate of 10% is established, the initiation of primary antifungal therapy in monotherapy should be avoided for the treatment of severe cases of IA/IPA. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [Aspergillus resistance to antifungal drugs]) Table 5 4,21,54,67,128,139,150.
97. It is recommended in the patient with a diagnosis of proven/probable IA/IPA, during primary targeted antifungal therapy, to perform TDM of the azoles (VCZ, ITZ, PCZ) of choice to improve the antifungal efficacy, evaluate therapeutic failure and decrease pharmacological toxicity. (strong recommendation, high-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Tables 9 and 10 21,151,152.
a. In the patient with proven/probable IA/IPA, what is the standard of care according to the clinical scenario?
Recommendation
98. Consensus recommends in the patient with a diagnosis of proven/probable IA/IPA the use of several antifungal drugs to initiate primary targeted antifungal therapy and increase response and survival rate. The azoles (VCZ, ISZ, ITZ: [standard dose]) IV., are the antifungal drugs of choice. It is considered that although ITZ is effective, however its use may be limited by its absorption and tolerabili-ty. (strong recommendation, high-quality evidence)Table 9, Annexes 1 and 2) 4,8,21,67,70.
99. The consensus recommends that in special patient populations (transplant recipients [adult and pediatric] with CF, on ECMO/critically ill) with a diagnosis of proven/ probable IA/IPA, the adjustment of antifungal drug doses should be performed in coordination with a hospital pharmacy specialist prior to the initiation of a primary targeted antifungal therapy. (strong recommendation, moderate-quality evidence)4,8,21,67,151.
i. ICU patient:
Recommendation
100. The consensus recommends in the ICU hospitalized patient with a diagnosis of proven/probable IA/IPA the initiation of primary targeted antifungal therapy. (strong recommendation, high-quality evidence)4,8,21,67,68,85.
101. The consensus considers that the diagnosis of certainty of a proven/probable IA/IPA is difficult, so it is considered the initiation of a targeted primary antifungal treatment in the ICU patient with high suspicion of developing an IFI and with the following conditions: (a) COPD, (b) structural pneumonia as an underlying disease, or (c) highdose corticosteroid therapy. (strong recommendation, moderate-quality evidence)Table 9 4,21,67,85,153.
Recommendation
102. The consensus recommends initiation of primary targeted antifungal therapy in HIV/AIDS patients with a diagnosis of proven/probable IA/IPA. It is considered that in patients with CD4 count <100 cells/mm 3 the choice of antifungal drug should be made on an individualized basis, evaluating possible drug-drug interactions with the antiretroviral drugs. (strong recommendation, highquality evidence) (Annexes 1 and 2) 4,21,67,86,154,155.
Recommendation
103. In the patient with a hematologic malignancy (AML/ MDS, in induction), and/or HSCT with/without profound and prolonged neutropenia, with a diagnosis of proven/ probable IA/IPA, the consensus recommends the initiation of primary targeted antifungal therapy. Delayed initiation of treatment is considered to be associated with worse clinical course, higher incidence of gap fungemia and elevated mortality. (strong recommendation, high-quality evidence)Table 9 4,21,67,68,128,134,156.
104. The consensus considers that in the patient with a hema-tologic malignancy (AML/MDS, in induction) and/or HSCT, with/without profound and prolonged neutropenia, the choice of drug for the initiation of primary targeted anti-fungal therapy will depend on: (a) the risk of IFI/IA, (b) the patient's characteristics and baseline disease, and (c) the type of associated treatment. (strong recommendation, high-quality evidence)Tables 2 and 4 4,8,21,67,68,128,134,153.
105. The consensus recommends in the HSCT patient in GVHD phase, with high suspicion and/or diagnosis of a proven/probable A//IPA, early initiation of primary targeted antifungal therapy to increase the response and survival rate. (strong recommendation, high-quality evidence)4,21,67,68,88,115,128.
1. Patient undergoing biologic therapy:
Recommendation
106. The consensus considers the immunocompromised patient undergoing treatment with TNF-a antagonist drugs (infliximab, adalimumab, etanercept) and/or anti-lymphocyte biologic agents (rituximab, alemtuzumab), has a high risk of developing an IFI/IA. A review of clinical history, previous fungal exposure and pathogenic determinants of infection is recommended prior to the initiation of primary targeted antifungal therapy. (strong recommendation, moderate-quality evidence)Table 2, Annex 7) 4,21,67,153,157.
107. The consensus considers in the immunocompromised patient undergoing treatment with TNF-a antagonist drugs (infliximab, adalimumab, etanercept) and/or with antilymphocyte biologic agents (rituximab, alemtuzumab), with a diagnosis of proven/probable IA/IPA, the initiation of primary targeted antifungal therapy. It is recommended to discontinue immunosuppressive drug during antifungal therapy and to evaluate its resumption once the invasive infection is controlled. (strong recommendation, moderate-quality evidence)Table 2, Annex 7) 4,21,67,153,157.
108. The consensus considers that in the immunocompromised patient the risk of IFI/IA does not depend exclusively on fungal exposure and/or treatment with immunosuppressi-ve drugs but on the possible joint effect with immunomo-dulatory drugs and/or chemotherapeutic agents administered simultaneously. (strong recommendation, moderate-quality evidence)Table 2, Annex 7) 4,21,67,153,157.
Recommendation
109. The consensus recommends in the SOTR patient with a high suspicion and/or diagnosis of proven/probable IA/ IPA, the early initiation of primary targeted antifungal therapy to increase the response and survival rate. An additional diagnostic approach is considered to establish proven/probable post-transplant IA/IPA. (strong recommendation, high-quality evidence)8,21,28,67,142,158.
110. It is considered that in the SOTR patient with a diagnosis of proven/probable IA/IPA, the choice of an antifungal drug for the initiation of primary targeted antifungal therapy should be made on an individualized basis, according to: (a) type of transplant, (b) severity of infectious disease, and (c) immunosuppressive regimen used. (strong recommendation, high-quality evidence) (Annexes 1 and 2) 4,8,21,28,67,158.
111. When choosing a drug for primary targeted antifungal therapy in a non-hematologic patient with a diagnosis of proven/probable IA/IPA, it is recommended to consider possible drug interactions due to the coadministration of immunosuppressive drugs. (strong recommendation, highquality evidence) (Annexes 1 and 2) 4,8,21,67.
112. It is recommended in the SOTR patient with a diagnosis of proven/probable IA/IPA to decrease (or increase) the dose of CNI/mTOR inhibitor administered, at the initiation and completion of primary azole-directed antifungal therapy and according to the concept of organ transplant, infectious disease and hospital pharmacy specialists. Monitoring CNI/mTOR inhibitor levels is considered. (strong recommendation, moderate-quality evidence) (Annexes 1 and 2) 4,8,21,67.
113. The consensus recommends in the SOTR patient with a diagnosis of proven/probable IA/IPA, receiving chronic antifungal treatment with an azole, to perform a baseline and follow-up electrocardiogram to evaluate the QT interval (in the patient receiving antifungal treatment with an azole, other than ISZ) and a regular skin examination (in the patient receiving VCZ). (strong recommendation, high-quality evidence) (Annexes 1 and 2) 4,8,21,67.
Recommendation
114. The consensus recommends in the lung transplant recipient patient with fungal colonization of the lower respiratory tract and/or diagnosis of invasive bronchial aspergillosis (IBA) (pseudomembranous tracheobronchitis or ulcerative tracheobronchitis) the initiation of primary targeted antifungal therapy. A FBC with BAL and chest multi-slice CT is considered to rule out an invasive process and/or dissemination. (strong recommendation, moderate-quality evidence)Tables 6 and 7 4,8,21,67.
b. In the patient with primary targeted antifungal therapy, what is the recommendation for the choice of drug type, dose and duration of anti-fungal therapy?
ICU patient
115. The consensus recommends the use of VCZ (IV., 6 mg/ kg/12h, day 1, then 4 mg/kg/12h) as the first option for primary targeted antifungal therapy in patients hospitalized in the ICU with a diagnosis of proven/probable IA/ IPA. A TDM is recommended to improve antifungal efficacy, evaluate therapeutic failure and decrease drug toxicity. (strong recommendation, high-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Tables 9 and 10, Annexes 4 and 5) 4,21,67,86,153,159.
116. It is considered that in the patient hospitalized in the ICU with a diagnosis of proven/probable IA/IPA, the duration of antifungal treatment should be established on an individualized basis, depending on the clinical and imaging evolution of the patient. (strong recommendation, moderate-quality evidence)4,8,21,67,68,85,153.
HIV/AIDS patient
117. The consensus recommends the use of VCZ (IV., 6 mg/ kg/12h, day 1, then 4 mg/kg/12h) as the first choice of primary targeted antifungal therapy in HIV/AIDS patients diagnosed with proven/probable IA/IPA. It is recommended to perform a TDM of the azoles (VCZ, PCZ, ITZ) of choice to improve antifungal efficacy, evaluate therapeutic failure and reduce drug toxicity. (strong recommendation, high-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Tables 9 and 10, Annexes 4 and 5) 4,21,67,86,153,159,160.
118. It is considered that in the HIV/AIDS patient with a diagnosis of proven/probable IA/IPA, the duration of antifungal treatment should be established on an individualized basis, depending on the clinical and imaging evolution of the patient. (strong recommendation, moderate-quality evidence)4,21,67,86,153.
Patient with hematologic malignancy and/or HSCT
119. The consensus recommends the use of VCZ (IV., 6 mg/ kg/12h, day 1, then 4 mg/kg/12h) or ISZ (IV., 200 mg/8h, day 1-2, then 200 mg/d) in patients with hematologic malignancy (AML/MDS, in induction) and/or allogeneic HSCT, with/without profound and prolonged neutropenia, with a diagnosis of proven/probable IA/IPA, as the first option of primary antifungal targeted therapy. It is recommended to perform a TDM of VCZ to improve antifungal efficacy, evaluate therapeutic failure and reduce drug toxicity. (strong recommendation, high-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Tables 9 and 10, Annexes 4 and 5) 4,21,67,68,70,88,159-162.
120. L-AmB (IV., 3-5 mg/kg/d) or LC-AmB (IV., 5 mg/kg/d), are an alternative for primary targeted antifungal therapy in the patient with hematologic malignancy (AML/MDS, in induction), and/or allogeneic HSCT, with/without profound and prolonged neutropenia, with a diagnosis of a proven/ probable IA/IPA, when there is a risk of: (a) hepatotoxicity from VCZ use, (b) intolerance or allergy from azole use, (c) drug-drug interactions. The consensus does not recommend the use of D-AmB for primary targeted antifungal therapy. (strong recommendation, moderatequality evidence)Table 9, Annexes 1 and 2) 4,21,67,68,70,88.
121. An echinocandin (IV., CAS [70 mg, day 1, then 50 mg/d], ANF [200 mg, day 1, then 100 mg/d], MCF [100 mg/d]), alone or in combination, may be considered for salvage antifungal therapy (or when azoles or polyenes are contraindicated and/or not tolerated) in the patient with hematologic malignancy (AML/MDS, in induction), and/or allogeneic HSCT, with/without profound and prolonged neutropenia, with a diagnosis of proven/probable IA/IPA. Routine use of an echinocandin in monotherapy, as primary antifungal therapy, is not recommended. (strong recommendation, moderate-quality evidence)Table 9, Annex 6) 4,8,21,67.
122. PCZ (IV., 300 mg/12h, day 1, then 300 mg/d), can be considered as an alternative for a salvage antifungal treatment in the patient with hematologic malignancy (AML/MDS, in induction), with/without profound and prolonged neutropenia, with a diagnosis of proven/probable IA/IPA, when there is a risk of: (a) hepatotoxicity from VCZ use, (b) drug-drug interactions, (c) treatment-refractory IA/IPA cases. (strong recommendation, moderate-quality evidence)Table 9 4,21,67,163,164.
Patient undergoing biological therapy
123. The consensus recommends the use of VCZ (IV., 6 mg/ kg/12h, day 1, then 4 mg/kg/12h) as the first option for primary targeted antifungal therapy in patients undergoing biologic therapy with a diagnosis of proven/probable IA/ IPA. TDM is recommended to improve antifungal efficacy, evaluate therapeutic failure and decrease drug toxicity. (strong recommendation, high-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Tables 9 and 10, Annexes 4 and 5) 4,21,157.
124. It is considered that in the patient undergoing biologic therapy, with a diagnosis of proven/probable IA/IPA, the duration of antifungal treatment should be established on an individual basis and should be a minimum of 6-12 months or for the duration of the immunosuppression. (strong recommendation, moderate-quality evidence)4,21,67,86,153.
SOTR patient
125. The consensus recommends in the SOTR patient with a diagnosis of proven/probable IA/IPA, as a first choice of primary targeted antifungal therapy the use of VCZ (IV., 6 mg/kg/12h, day 1, then 4 mg/kg/12h). TDM is recommended to improve antifungal efficacy, evaluate therapeutic failure and decrease drug toxicity. (strong recommendation, high-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Tables 9 and 10, Annexes 4 and 5) 4,8,21,67,159-161,165.
126. ISZ(IV., 200 mg/8h, day 1-2, then 200 mg/d) can be considered as an alternative for primary antifungal treatment, in the SOTR patient with a diagnosis of proven/probable IA/IPA. (strong recommendation, moderate-quality evidence)Table 9 4,21,67,70.
127. L-AmB (IV., 3-5 mg/kg/d) is an alternative for primary targeted antifungal therapy in the SOTR patient with a diagnosis of proven/probable IA/IPA, when there is a risk of: (a) hepatotoxicity from VCZ use, (b) intolerance or allergy from azole use, and/or (c) drug-drug interactions. The use of D-AmB for primary antifungal therapy is not recommended and the possible associated nephrotoxi-city (particularly in kidney transplant recipients) should be considered. (strong recommendation, moderate-quality evidence)Table 9, Annexes 1 and 2) 4,8,21,67,68.
128. An echinocandin (IV., CAS [70 mg, day 1, then 50 mg/d], ANF [200 mg, day 1, then 100 mg/d], MCF [100 mg/d]), alone or in combination, may be considered for salvage antifungal therapy (or when contraindicated and/or azoles or polyenes are not tolerated) in the SOTR patient with a diagnosis of proven/probable IA/IPA. Routine use of an echinocandin in monotherapy as primary antifungal therapy is not recommended. (strong recommendation, moderate-quality evidence)Table 9, Annex 6) 4,8,21,67,166,167.
129. PCZ (IV., 300 mg/12h, day 1, then 300 mg/d) can be considered as an alternative for salvage antifungal therapy in the SOTR patient with a diagnosis of proven/probable IA/IPA, when there is a risk of: (a) hepatotoxicity from VCZ use, (b) drug-drug interactions, (c) treatment-refractory IA/IPA cases. (strong recommendation, moderate-quality evidence)Table 9 4,21,67,163,168,169.
130. It is considered that in the SOTR patient with a diagnosis of proven/probable IA/IPA, the duration of antifungal treatment should be at least 12 weeks. The duration will depend on the clinical and imaging response of the patient. (strong recommendation, moderate-quality evidence)4,8,21,67,165.
SOTR patient - Lung transplant recipient
131. It is recommended in the lung transplant recipient patient with an AGA result > 1, from BAL, the initiation of a primary targeted antifungal treatment with PCZ (IV., 300 mg/12h, day 1, then 300 mg/d), or VCZ (IV., 6 mg/ kg/12h, day 1, then, 4 mg/kg/12h). It is recommended to perform a TDM of the azoles (VCZ, PCZ) of choice to improve antifungal efficacy, evaluate therapeutic failure and decrease pharmacological toxicity. (strong recommendation, moderate-quality evidence)Tables 9 and 10 4,8,21,67,68,115,128,159.
132. The consensus recommends in the lung transplant recipient patient with a diagnosis of IBA (pseudomembranous tracheobronchitis or ulcerative tracheobronchitis), initiation of primary targeted antifungal therapy with an azole (VCZ, ISZ, ITZ: [standard dose]) or a lipid formulation of AmB (L-AmB, LC-AmB: [standard dose]). Consideration is given, if feasible, to minimizing or reversing underlying immunosuppression along with careful risk assessment and in selected cases, bronchoscopic debridement of the airway lesions. (strong recommendation, moderate-quality evidence)Table 11 4,8,21,67.
Table 11 Adjuvant surgery for the management of an IA.
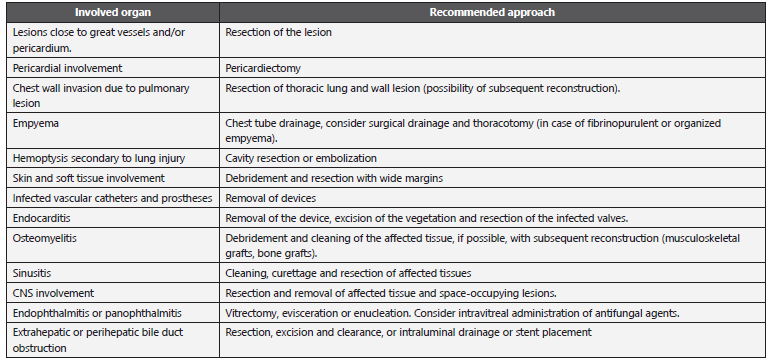
CNS: Central nervous system.
Adapted from: Fortún J et al. 20; García-Vidal C et al. 67; Walsh TJ et al. 148.
133. The consensus recommends, in the lung transplant recipient patient with fungal colonization of the lower respiratory tract and/or diagnosis of IBA (pseudomembranous tracheobronchitis or ulcerative tracheobronchitis), in the context of anastomotic endobronchial ischemia or ischemic reperfusion injury (due to transplant-associated airway ischemia), the complementary use of nebulized L-AmB. It is considered that the duration of antifungal treatment should be at least 3 months or until complete resolution of the lesions. (strong recommendation, low-quality evidence)4,8,21,67.
SOTR patient - Heart transplant recipient
134. ITZ (IV., 200 mg/12h, day 1-2, then 200 mg/d) can be considered as an alternative for primary targeted anti-fungal therapy in the heart transplant recipient patient with a diagnosis of proven/probable IA/IPA. Consideration should be given when using such a drug to: (a) inadequate absorption, (b) a narrow therapeutic window, and (c) potential drug interactions and/or unspecified toxicity. (weak recommendation, low-quality evidence) (Annexes 1 and 2) 8,21.
Recommendation
135. It is recommended in the patient with a diagnosis of proven/probable IA/IPA, with localized and easily accessible involvement (e.g., invasive sinusitis and/or cutaneous) the initiation of primary targeted antifungal therapy associated with adjunctive surgical management with surgical debridement. Surgical benefit in other settings (endocarditis, osteomyelitis and/or focal CNS disease) is considered to be established on an individualized basis andreview of: (a) immunologic status of the patient, (b) comorbidities, (c) confirmation of a single focus, and (d) surgical risk. (strong recommendation, moderate-quality evidence)Tables 6 and 11 4,21,67,170.
136. The consensus recommends in the patient with hematologic malignancy (AML/MDS, on induction), with/without profound and prolonged neutropenia, with a diagnosis of proven/probable IA/IPA and/or life-threatening hemoptysis, careful risk assessment followed by arterial embolization and emergency surgical intervention. (strong recommendation, moderate-quality evidence)Tables 2 and 11 4,21,67,170,171.
Recommendation
137. In the patient with a hematologic malignancy (AML/ MDS, in induction) and/or HSCT with/without profound and prolonged neutropenia, with a diagnosis of proven/ probable IA/IPA, the consensus recommends, when feasible, tapering and/or discontinuation of immunosuppres-sive agents as an adjunct to initiation of primary targeted antifungal therapy4,21,67,68,70,88.
138. In the SOTR patient with a diagnosis of proven/probable IA/IPA, it is recommended, when feasible, dose reduction and/or discontinuation of immunosuppressive agents as an adjunctive to the initiation of primary targeted anti-fungal therapy but without threatening the graft outcome. Lowering the dose of corticosteroids is considered to be the preferred approach. (strong recommendation, moderate-quality evidence)8,67.
139. The consensus recommends in the patient with a diagnosis of proven/probable IA/IPA and severe disease and/or failure and/or refractoriness to primary targeted antifungal therapy, the adjunctive use of interferon gamma (IFN-y) even though it has not clearly demonstrated a therapeutic benefit. Its use is not considered to be associated with worsening of the GVHD phase in allogeneic HSCT patients or in allograft rejection in the SOTR patient. (strong recommendation, low-quality evidence)4,21,67,68,70,88.
2. What is the consideration for the addition of colony stimulating factors (CSF) or granulocyte transfusions?
Recommendation
140. The consensus suggests in the patient with hematologic malignancy (AML/MDS, in induction) and/or HSCT with profound and prolonged neutropenia, with a diagnosis of proven/probable IA/IPA, to routinely avoid administration of granulocyte transfusions. Evidence is insufficient to recommend the use of granulocyte colony-stimulating factor (G-CSF-G) versus granulocyte-macrophage colony-stimulating factor (GM-CSF) in this setting. (weak recommendation, low-quality evidence)4,21,67,172-174.
141. The consensus suggests in the patient with hematologic malignancy (AML/MDS, in induction) and/or HSCT with profound and prolonged neutropenia, with a diagnosis of proven/probable IA/IPA, the administration of CSF and/or granulocyte transfusions. Their administration is considered in case of: (a) patient with progressive IFI/IA associated with failure and/or refractoriness and/or (b) patient with neutropenia > 7 days with low probability of response to standard antifungal therapy. There is no evidence that the administration of CSF and/or granulocyte transfusions decreases mortality associated with invasive disease. (weak recommendation, low-quality evidence)4,2,67,68,70,88,172-175.
Recommendation
142. The consensus recommends that in the patient with hematologic malignancy (AML/MDS, in induction) with profound and prolonged neutropenia, with a diagnosis of proven/probable IA/IPA, the decision to initiate additional chemotherapy and/or HSCT should involve multidisciplinary therapeutic management and close consultation with hematology/oncology and infectious diseases specialists. The risk of progressive IA during the period following antineoplastic treatment should be considered versus the risk of death from the underlying malignancy if such treatment is delayed. (strong recommendation, lowquality evidence)4,175-178.
3. In the patient with proven/probable IA/IPA, what is the therapeutic management approach for refractory/progressive aspergillosis (salvage antifungal therapy)?
Recommendation
143. The consensus considers that in the patient with a diagnosis of proven/probable IA/IPA and progressive disease, associated with failure and/or refractoriness to primary targeted antifungal therapy, it is due to: (a) the type of patient (severe baseline disease and/or persistent immunodeficiency), (b) an initial incorrect diagnosis (failure to identify or incorrect identification of the responsible species and/or its resistance profile and/or TDM of the drugs in use), (c) coexistence of other infectious processes, and (d) low concentration of the antifungal drug of choice at the involved site (e.g., necrotic tissue). (strong recommendation, high-quality evidence)4,21,67,153,79-184.
144. The consensus recommends in the patient with a diagnosis of proven/probable IA/IPA, the initiation of salvage antifungal therapy when primary antifungal therapy is refractory and/or not tolerated after a follow-up of ± 7 days. (strong recommendation, high-quality evidence)3,4,21,67,128,134-136,179-185
145. The consensus considers that in the patient with a diagnosis of proven/probable IA/IPA and progressive disease associated with failure and/or refractoriness to primary targeted antifungal therapy, the decision to initiate salvage antifungal therapy should be made on an individualized basis. (strong recommendation, moderate-quality evidence)Table 9, Annexes 1 and 2) 4,67,182-184.
a. In the patient with refractory/progressive asper-gillosis (salvage antifungal therapy), what is the recommendation for the choice of drug type, dosage and duration of antifungal therapy?
Recommendation
146. The consensus considers that in the patient with a diagnosis of proven/probable IA/IPA the approach to initiation of salvage antifungal therapy includes: (a) change of primary antifungal drug class and/or addition of another antifungal drug to primary therapy, (b) use of an antifungal drug with an adverse effect profile that does not overlap with other co-administered drugs, (c) decrease or reversal of underlying immunosuppression (if feasible), and (d) surgical resection of necrotic lesions (in selected cases). (strong recommendation, moderate-quality evidence)Table 9, Annexes 1 and 2) 4,67,182-184.
147. The consensus recommends in the patient with a diagnosis of proven/probable IA/IPA and progressive disease, the initiation of salvage antifungal therapy to achieve a complete or partial response and improve survival. The azoles (VCZ, ISZ, PCZ, ITZ), lipid formulations of AmB and/or an echinocandin (CAS, ANF, MCF) IV are the drugs of choice for the therapeutic management of refractory disease. Consideration should be given when using an azole drug to: (a) administered prophylaxis or previous treatment, (b) patient risk factors, (c) pharmacokinetic considerations of the drug of choice, and (d) possible manifestation of anti-fungal resistance. (strong recommendation, moderate-quality evidence)Table 9, Annexes 1,2 and 6) 4,8,21,67,70.
Patient with hematologic malignancy and/or HSCT
148. The consensus recommends in the patient with hemato-logic malignancy (AML/MDS, in induction) and/or HSCT with/without profound and prolonged neutropenia, with a diagnosis of proven/probable IA/IPA in whom refractoriness to primary antifungal therapy is suspected, the initiation of salvage antifungal therapy, in monotherapy or in combination with: (a) VCZ (IV., 6 mg/kg/12h, day 1, then, 4 mg/kg/12h), (b) PCZ (IV., 300 mg/12h, day 1, then 300 mg/d), (c) AMB-L (IV., 3-5 mg/kg/d), (d) LC-AmB (IV., 5 mg/kg/d), or (e) an echinocandin (IV., CAS [70 mg, day 1, then 50 mg/d], ANF [200 mg, day 1, then 100 mg/d], MCF [100 mg/d]). (strong recommendation, moderate-quality evidence) (Annex 6) 4,21,67,179-181.
4. In the patient with proven/probable IA/IPA, what is the recommendation for the choice of antifun-gal treatment in combination according to the at-risk population?
Recommendation
149. The consensus does not recommend in the patient with a diagnosis of proven/probable IA/IPA the routine choice of antifungal combination therapy. (strong recommendation, moderate-quality evidence) (Annex 6) 4,8,21,67,153,186-191.
150. The consensus considers that in the patient with a diagnosis of proven/probable IA/IPA the conditions that favor the choice of antifungal combination therapy are: (a) high-risk patient, when the isolated Aspergillus species is unknown, (until AFST results, are available), (b) patient in whom the loading dose of VCZ was not administered and/or is expected to be highly influenced by concomitant drugs (until TDM results are available), (c) patient on salvage antifungal therapy due to failure of primary antifun-gal monotherapy, (d) patient with CNS involvement or a severe disseminated form (e.g., a severe form of VCZ) (e.g., a patient with a severe form of VCZ (e.g., a severe form of VCZ), sepsis or multi-organ dysfunction), (e) severely immunosuppressed patient (HSCT, SOTR, HIV [ CD4 <100 cells/mm3]), (f) transplanted patient with increased risk factors (renal failure, GVHD, high doses of corticosteroids, treatment with TNF-a antagonist), or (g) patient with pulmonary infection and respiratory failure or bilateral, extensive, cavitated lesion. (strong recommendation, moderate-quality evidence) (Annex 6) 4,8,21,54,67,139,186-192.
A. In the patient with proven/probable IA/IPA, what is the recommendation for the choice of drug type, dose and duration of antifungal combination therapy?
Patient with hematologic malignancy and/or HSCT
151. VCZ (IV., 6 mg/kg/12h, day 1, then, 4 mg/kg/12h) and an echinocandin (IV., CAS [70 mg, day 1, then 50 mg/d], ANF [200 mg, day 1, then 100 mg/d], MCF [100 mg/d]) can be considered for combination antifungal treatment in the patient with hematologic malignancy (AML/MDS, on induction) and/or allogeneic HSCT, with/without profound and prolonged neutropenia, with a diagnosis of proven/ probable IA/IPA in whom failure and/or refractoriness and/or development of antifungal resistance to primary antifungal therapy is suspected. (strong recommendation, low-quality evidence)Table 5, Annex 6) 4,21,67,68,70,88.
152. AmB (IV., L-AmB [3-5 mg/kg/d] or LC-AmB [5 mg/kg/d]) and an echinocandin (IV., CAS [70 mg, day 1, then 50 mg/d], ANF [200 mg, day 1, then 100 mg/d], MCF [100 mg/d]) can be considered for combination antifungal treatment in the patient with hematologic malignancy (AML/MDS, on induction) and/or allogeneic HSCT, with/without profound and prolonged neutropenia, with a diagnosis of proven/probable IA/IPA in whom failure and/or refractoriness and/or development of antifungal resistance to primary antifungal therapy is suspected. (strong recommendation, low-quality evidence)Table 5, Annex 6) 4,21,67,68,70,88.
SOTR patient
153. It is recommended that in the SOTR patient diagnosed with severe forms of IA (e.g., CNS involvement and/or disseminated disease), consideration be given to initiating combination antifungal therapy at least until an optimal therapeutic concentration of VCZ is achieved. (strong recommendation, low-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Tables 6 and 10, Annexes 5 and 6) 8,67,193.
154. VCZ (IV., 6 mg/kg/12h, day 1, then, 4 mg/kg/12h) and an echinocandin (IV., CAS [70 mg, day 1, then 50 mg/d], ANF [200 mg, day 1, then 100 mg/d], MCF [100 mg/d]) may be considered for primary combination antifungal therapy in the SOTR patient with a diagnosis of proven/ probable IA/IPA, in whom the development of azole resistance and/or renal failure associated with primary anti-fungal therapy is suspected. (strong recommendation, low-quality evidence)Table 5, Annex 6) 4,21,67,68,70,88.
SECTION IV: DIAGNOSIS AND THERAPEUTIC MANAGEMENT OF IA IN THE PEDIATRIC AND NEONATAL PATIENT
1. In the pediatric patient with a high suspicion of developing an IFI/IA, how is the diagnostic approach performed?
Imaging study
155. The consensus recommends in the pediatric patient with high suspicion of developing an IFI, to perform a multi-slice CT of the chest in order to perform the diagnostic approach of a proven/probable IA/IPA, in the presence of: (a) profound and prolonged neutropenia, (b) presence of characteristic clinical symptoms (pleuritic chest pain, blood-tinged sputum and/or hemoptysis), (c) presence of suggestive clinical signs (new-onset pneumonia, tenderness, or edema around the sinuses or orbital area, ulcera-tive lesions or eschar in the nasal area), (d) positive culture for Aspergillus spp. from sputum, or (e) positive AGA and/or PCR test from serum. (strong recommendation, high-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [imaging approach for the diagnosis of IPA]) Table 7 132,136,192,194-197.
156. The consensus does not recommend performing a chest CT with contrast in pediatric patients with a high suspicion of developing an IA/IPA. A chest CT with contrast is recommended in the presence of a nodule/mass close to a large vessel. (strong recommendation, moderate-quality evidence)Table 7 129,136,195-197.
157. The consensus does not recommend in the pediatric patient with hematologic malignancy and profound and prolonged neutropenia taking a chest X-ray to perform the diagnostic approach of a proven/probable IA/IPA. (strong recommendation, moderate-quality evidence)136,195-197.
158. The consensus recommends in the pediatric patient with SOTR or chronic granulomatous disease (CGD) taking a chest X-ray to perform the diagnostic approach of a proven/probable IA/IPA. (weak recommendation, moderate-quality evidence)136,195-197.
159. The consensus recommends in the pediatric patient with a diagnosis of IA/IPA taking a follow-up chest CT scan to evaluate the response to antifungal therapy after a minimum of 2 weeks of treatment. More frequent follow-up is recommended if the patient deteriorates clinically and/or in the presence of a nodule/mass near a large vessel. (strong recommendation, high-quality evidence)196-198.
160. In the pediatric patient, a CT scan of the paranasal sinuses is considered when there is a suspicion of sinus involvement, in order to perform the diagnostic approach of a proven/probable IA. The consensus does not recommend in the pediatric patient with high suspicion of an IFI to routinely perform a CT scan of the paranasal sinuses. (weak recommendation, low-quality evidence)147,199,200.
161. The consensus recommends in the pediatric patient with risk factors and neurological symptoms taking a brain MRI with contrast upon suspicion of CNS involvement, to perform the diagnostic approach of a proven/probable IA. (strong recommendation, moderate-quality evidence)199,201-206.
Fungal biomarkers
162. The consensus recommends in the high-risk pediatric patient with hematologic malignancy and/or HSCT, with/ without profound and prolonged neutropenia, who are not on antifungal prophylaxis or treatment, the use of the AGA test from serum with serial monitoring (x3/wk) as a screening and diagnostic test for proven/probable IA. (strong recommendation, high-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [usefulness of AGA and BDG for disease diagnosis]) (Annex 3) 194,207.
163. The consensus does not recommend in the high-risk pediatric patient with hematologic malignancy and/ or HSCT, with/without profound and prolonged neutropenia, who are on antifungal prophylaxis or treatment the use of serum-based AGA as an early detection test for proven/probable IA. Measurement of AGA from BAL is considered for the diagnostic approach of proven/probable IA/IPA. (strong recommendation, high-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [usefulness of AGA and BDG for disease diagnosis]) (Annex 3) 194,207-209.
164. The consensus does not recommend in the high-risk neonatal and the high-risk SOTR and CGD pediatric patient, the measurement of AGA from serum for the diagnostic approach of proven/probable IA. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [usefulness of AGA and BDG for disease diagnosis]) (Annex 3) 207-209.
165. The consensus does not recommend in the pediatric patient the measurement of BDG from serum as a screening and diagnostic test for proven/probable IA. (weak recommendation, low-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [usefulness of AGA and BDG for disease diagnosis]) (Annex 3) 209,210.
166. The consensus recommends in the high-risk pediatric patient with hematologic malignancy and/or HSCT, with/ without profound and prolonged neutropenia, the detection of fungal DNA by PCR-Aspergillus test from blood, serum and/or BAL for the diagnostic approach of proven/ probable IA. (weak recommendation, low-quality evidence) (I Diagnosis and Followup of IA/Aspergillus Disease [usefulness of nucleic acid testing and mass spec-trometry for the disease diagnosis]) (Annex 3) 67,209-216.
a. In the pediatric patient with a diagnosis of proven/probable IA/IPA, what are the recommended antifungal treatment regimens?
Recommendation
167. The consensus recommends in the pediatric patient older than 2 years of age, with a diagnosis of proven/probable IA/IPA, the use of VCZ (IV., 2-12 years or 12-14 years and weight < 50 kg, 9 mg/kg/12h, one dose, then 8 mg/ kg/12h) as a first choice of antifungal treatment. TDM is recommended to improve antifungal efficacy, evaluate therapeutic failure and decrease drug toxicity. (strong recommendation, high-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Tables 9 and 10 151,194,200,217-224.
168. L-AmB (3-5 mg/kg/d) is an alternative for antifungal treatment in pediatric patients older than 2 years of age with a diagnosis of proven/probable IA/IPA. (weak recommendation, low-quality evidence)Table 9 194,200,27,218.
169. It is considered that in the pediatric patient with a diagnosis of proven/probable IA/IPA, the duration of antifungal treatment should be established on an individualized basis and should be a minimum of 4-6 weeks. (strong recommendation, high-quality evidence)194,200,217,218.
2. In the pediatric patient with a diagnosis of an IFI/ IAI, what is the recommendation for the choice of drug type, dosage and duration of antifungal treatment according to the clinical scenario?
Recommendation
170. In the critically ill pediatric patient older than 2 years of age, with a diagnosis of proven/probable IA, the consensus recommends as a first antifungal treatment option the use of VCZ (IV., 2-12 years or 12-14 years and weight < 50 kg, 9 mg/kg/12h, one dose, then 8 mg/kg/12h). TDM is recommended to improve antifungal efficacy, evaluate therapeutic failure and decrease drug toxicity. (strong recommendation, high-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/Aspergillus disease]) Tables 9 and 10 4,151,194,217,218.
171. L-AmB (3-5 mg/kg/d) is an alternative for antifungal treatment in the critically ill pediatric patient older than 2 years of age with a diagnosis of proven/probable IA. (weak recommendation, low-quality evidence)Table 9 149,194,200,217,223,225.
172. An echinocandin (CAS [> 3 months 70 mg/m2, then 50 mg/ m2/d, one dose, not to exceed adult dose], ANF [3 mg/kg, one dose, then 1,5 mg/kg/d], MCF [> 4 months (<40 kg): 2-4 mg/kg/d in one dose; > 40 kg: 100 mg/d]), alone or in combination, may be considered for salvage antifungal therapy in the critically ill pediatric patient with a diagnosis of proven/probable IA. (weak recommendation, low-quality evidence) (section: targeted antifungal treatment of IA/IPA [therapeutic management approach to refractory/ progressive aspergillosis]) Table 9, Annex 6) 194,218.
173. In the critically ill pediatric patient older than 2 years of age with a diagnosis of proven/probable IA, the consensus does not recommend the initiation of antifungal treatment with nebulized AmB. (weak recommendation, low-quality evidence)4,194.
Recommendation
174. The consensus recommends the use of VCZ (IV., 2-12years or 12-14 years and weight < 50 kg, 9 mg/kg/12h, one dose, then 8 mg/kg/12h) as the first antifungal treatment option in the pediatric patient with HIV/AIDS infection, with a diagnosis of proven/probable IA. TDM is recommended to improve antifungal efficacy, evaluate therapeutic failure and decrease drug toxicity. (strong recommendation, high-quality evidence) (section: targeted antifungal treatment of IA/IPA [HIV/AIDS patient]) Tables 9 and 10, Annexes 4 and 5) 4,21,151,194,218,220,221,226.
Recommendation
175. The consensus recommends the initiation of primary, universal and/or targeted antifungal prophylaxis (PCZ, VCZ) in pediatric patients with a high suspicion of developing an IFI/IA. The populations that, according to their specific clinical condition, are recognized for the initiation of primary antifungal prophylaxis are: (a) allogeneic HSCT pre-graft phase, (b) allogeneic HSCT in post-graft phase, (c) HSCT in GVHD phase and increased immunosuppression, (d) high risk patients with de novo or recurrent leukemia, and (e) patients with bone marrow failure syndrome with profound and prolonged neutropenia. (strong recommendation, high-quality evidence)Table 9 21,147,194.
176. In the pediatric patient with hematologic malignancy and/or allogeneic HSCT, with/without profound and prolonged neutropenia, with diagnosis of a proven/probable IA, the consensus recommends as first choice of antifun-gal treatment the use of VCZ (IV., 2-12 years or 12-14 years and weight < 50 kg, 9 mg/kg/12h, one dose, then 8 mg/kg/12h). TDM is recommended to improve antifungal efficacy, evaluate therapeutic failure and decrease drug toxicity. (strong recommendation, high-quality evidence) (section: targeted antifungal treatment of IA/IPA [patient with hematologic malignancy/HSCT]) Tables 9 and 10, Annexes 4 and 5) 4,21,147,151,194,200,218,221,226.
177. L-AmB (3-5 mg/kg/d) is an alternative for antifungal therapy in the pediatric patient with hematologic malignancy and/or allogeneic HSCT, with/without profound and prolonged neutropenia, with a diagnosis of proven/probable IA. (weak recommendation, moderate quality evidence)Table 9 147,149,194,200,217,223,225.
178. PCZ (suspension [200 mg/8h] or tablets [300 mg/12h, two doses, then 300 mg/d]) can be considered as an alternative for salvage antifungal therapy in the pediatric patient with hematologic malignancy and/or allogeneic HSCT, with/without profound and prolonged neutropenia, with a diagnosis of proven/probable IA. (weak recommendation, low-quality evidence)Table 9 147,194,218.
179. An echinocandin (CAS [> 3 months 70 mg/m2, then 50 mg/m2/d, one dose, not to exceed adult dose], ANF [3 mg/kg, one dose, then 1,5 mg/kg/d], MCF [> 4 months (<40 kg): 2-4 mg/kg/d in one dose; > 40 kg: 100 mg/d]) may be considered as an alternative for an antifungal treatment in the pediatric patient with hematologic malignancy and/or allogeneic HSCT, with/without profound and prolonged neutropenia, with a diagnosis of proven/ probable IA. (weak recommendation, low-quality evidence)Table 9, Annex 6) 147,194,218.
180. L-AmB and an echinocandin, or VCZ and an echinocandin may be considered for combination antifungal therapy in the pediatric patient with hematologic malignancy and/or allogeneic HSCT, with/without profound and prolonged neutropenia, with a diagnosis of proven/probable IA. (weak recommendation, low-quality evidence)Table 9, Annex 6) 147,194,218.
Recommendation
181. In the pediatric patient with a diagnosis of proven/probable IA, undergoing biologic therapy, the consensus recommends as a first antifungal treatment option the use of VCZ (IV., 2-12 years or 12-14 years and weight < 50 kg, 9 mg/kg/12h, one dose, then 8 mg/kg/12h). TDM is recommended to improve antifungal efficacy, evaluate therapeutic failure and decrease drug toxicity. (strong recommendation, high-quality evidence) (section: targeted antifungal treatment of IA/IPA [patient undergoing biologic therapy]) Tables 9 and 10, Annexes 4 and 5) 21,151,194,221,226,227.
182. A multidisciplinar therapeutic management is recommended in pediatric patients undergoing biologic therapy with a diagnosis of proven/probable IA, where the choice of antifungal drug should be individualized evaluating possible drug-drug interactions. (strong recommendation, high-quality evidence) (Annexes 1 and 2) 227.
183. It is considered that in the pediatric patient undergoing biologic therapy, with a diagnosis of proven/probable IA, the duration of antifungal treatment should be established on an individualized basis and should be a minimum of 6-12 months or for the duration of immunosuppres-sion. (strong recommendation, low-quality evidence) (section: targeted antifungal treatment of IA/IPA [patient undergoing biologic therapy]) 227.
Recommendation
184. The consensus recommends in the pediatric SOTR patient with high suspicion of developing an IFI/IA, early initiation of primary targeted antifungal therapy. (weak recommendation, moderate-quality evidence)4,8,194
185. The consensus considers that in the pediatric SOTR patient with a diagnosis of proven/probable IA, the choice of antifungal drug for the initiation of primary targeted antifungal therapy should be made on an individualized basis, according to: (a) type of transplant, (b) severity of infectious disease, and (c) immunosuppressive regimen used. (strong recommendation, moderate-qualityevidence)4,8,194.
186. In the pediatric SOTR patient with a diagnosis of proven/ probable IA, the consensus recommends as a first anti-fungal treatment option the use of VCZ (IV., 2-12 years or 12-14 years and weight < 50 kg, 9 mg/kg/12h, one dose, then 8 mg/kg/12h). TDM is recommended to improve antifungal efficacy, evaluate therapeutic failure and decrease drug toxicity. (strong recommendation, high-quality evidence) (section: targeted antifungal treatment of IA/IPA [SOTR patient]) Tables 8 and 9, Annexes 4 and 5) 8,21,151,194,221,223,226.
187. L-AmB (3-5 mg/kg/d), is an alternative for antifungal treatment in the pediatric SOTR patient with a diagnosis of proven/probable IA, when there is a risk of: (a) he-patotoxicity due to VCZ use, (b) drug-drug interactions, (c) intolerance to the use of azole therapy. Their nephro-toxic potential should be considered, especially in kidney transplant recipients. (strong recommendation, low-quality evidence).Table 9, Annexes 1 and 2) 4,194.
188. An echinocandin (CAS [> 3 months 70 mg/m2, then 50 mg/m2/d, one dose, not to exceed adult dose], ANF [3 mg/kg, one dose, then 1,5 mg/kg/d], MCF [> 4 months (<40 kg): 2-4 mg/kg/d in one dose; > 40 kg: 100 mg/d]), alone or in combination, may be considered as an alternative for an antifungal treatment in the pediatric SOTR patient with a diagnosis of proven/probable IA. (strong recommendation, low-quality evidence)4,194.
189. L-AmB and an echinocandin or VCZ and an echinocandin may be considered for combination antifungal therapy in the pediatric SOTR patient with a diagnosis of proven/ probable IA. (weak recommendation, low-quality evidence)Table 9, Annex 6) 4,167,223,224.
190. It is recommended in the pediatric SOTR patient diagnosed with a severe form of IA (e.g., CNS or disseminated involvement) to consider initiation of combination antifungal therapy. (weak recommendation, moderate-quality evidence) (section: targeted antifungal treatment of IA/IPA [combination antifungal therapy]) Tables 6 and 9, Annex 6) 4,167,194,223,224,228,229.
191. In the pediatric SOTR patient with a diagnosis of proven/ probable IA, tapering of the total amount of immunosup-pression is recommended, if feasible, as an adjunct to initiating primary targeted antifungal therapy but without threatening graft outcome. Lowering the dose of corticosteroids is considered to be the preferred approach. (strong recommendation, moderate-quality evidence)4,167,218,223,224.
Recommendation
192. In the pediatric patient with a primary immunodeficiency, especially patient with CGD with high suspicion of developing an IFI/IA, and according to their specific clinical condition, the consensus recommends the initiation of primary antifungal prophylaxis, universal and/or targeted, (VCZ, PCZ, ITZ) against filamentous fungi. (strong recommendation, high-quality evidence)Table 9 21,194.
193. In the pediatric patient with a primary immunodeficiency, especially patient with CGD, with a diagnosis of proven/ probable IA, the consensus recommends as a first choice of antifungal treatment for IA the use of VCZ (IV., 2-12 years or 12-14years and weight < 50 kg, 9 mg/kg/12h, one dose, then 8 mg/kg/12h). TDM is recommended to improve antifungal efficacy, evaluate therapeutic failure and decrease drug toxicity. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [TDM in therapeutical management of IA/ Aspergillus disease]) Tables 9 and 10 4,21,151,221,226,230-235.
194. L-AmB (3-5 mg/kg/d) is an alternative for antifungal treatment in the pediatric patient with a primary immunodeficiency, especially CGD patient, with a diagnosis of proven/probable IA, when there is a risk of: (a) hepatotoxicity due to the use of VCZ, (b) drug-drug interactions, (c) intolerance to the use of azole treatment. (strong recommendation, lowquality evidence)Table 9, Annexes 1 and 2) 4,194,230-235.
195. PCZ (suspension [200 mg/8h] or TLR [300 mg/12h, two doses, then 300 mg/d]) can be considered as an alternative for antifungal treatment in the pediatric patient with a primary immunodeficiency, especially patient with CGD, with a diagnosis of proven/probable IA. (weak recommendation, low-quality evidence)Table 9 218.
3. In the neonatal patient with a diagnosis of an IFI/IA, what is the recommendation for the choice of drug type, dosage and duration of antifungal treatment, according to the clinical scenario?
Recommendation
196. The consensus recommends the use of L-AmB (3-5 mg/ kg/d) as the first antifungal treatment option in the neonatal patient with a diagnosis of proven/probable IA. (strong recommendation, moderate-quality evidence)194,236-239.
197. D-AmB (0.4-1 mg/kg/d) is an alternative antifungal treatment in neonatal patients with a diagnosis of proven/probable IA. (weak recommendation, low-qualityevidence)Table 9 194,240-242.
198. LC-AmB (3-5 mg/kg/d), an echinocandin (CAS [<3 months of age, 25 mg/m2/d, one dose], MCF [4-10 mg/kg/d in one dose]) or compassionate use of VCZ may be considered as an alternative for an antifungal treatment in the neonatal patient with a diagnosis of proven/probable IA, in settings of limited resources or availability. (weak recommendation, low-quality evidence)Table 9 243-246.
PREVENTION OF INFECTIONS ASSOCIATED WITH Aspergillus spp. AND CONSIDERATIONS FOR THE IMPLEMENTATION OF ANTIFUNGAL STEWARDSHIP (AFS) PROGRAMMES
QUESTIONS:
1. In non-pharmacological prevention of Aspergillus spp. associated infections, what special considerations should be taken into account?
Recommendation
199. It is recommended in the severely immunocompromised patient, during the periods and/or episodes of increased risk (profound and prolonged neutropenia, transplantation, major surgery, etc.), as a strategy for the prevention of infections associated with Aspergillus spp., to implement environmental control measures to minimize environmental exposure to filamentous fungal conidia. (strong recommendation, high-quality evidence)4,21,247-257.
200. The consensus recommends having knowledge of the epidemiology of each hospital center as a measure of prevention of infections associated with Aspergillus spp. It is recommended to keep a record of cases of probable/ proven IFI to detect an increase in the incidence and/ or manifestation of hospital outbreaks. (strong recommendation, high-quality evidence)30,139,254,258,259.
a. In non-pharmacological prevention of Aspergillus spp. associated infections, what is the recommendation according to the population at risk?
Recommendation
201. he consensus recommends that healthcare personnel caring for high-risk immunocompromised patients receive specific training in fungal epidemiology, mechanisms of disease transmission, along with prevention and control measures. (strong recommendation, high-quality evidence)19,21,30,259-261.
202. The consensus recommends implementing strategies for the maintenance of hospital engineering and/or architecture in order to minimize environmental exposure to filamentous fungal conidia. It is considered that their design and evaluation should involve the responsible engineers and architects, the infection control team, and hospital management. (strong recommendation, high-quality evidence)251,262-265.
203. The consensus recommends for the high-risk severely immunocompromised patient, during their hospital stay, to provide them with a protected environment setting (PES) as a measure to prevent filamentous fungal-associated infections. (strong recommendation, high-quality evidence) (Annex 8) 19,21,30,259-261,263,264.
b. In non-pharmacological prevention of Aspergillus spp. associated infections, what are the sources of exposure to Aspergillus spp, and how can fungal exposure be reduced?
Recommendation
204. The consensus recommends for the high-risk severely im-munocompromised patient, such as the patient receiving an induction/reinduction regimen for hematologic malignancy and/or allogeneic HSCT, with/without profound and prolonged neutropenia during his or her hospital stay, to provide him or her with an PES as a measure of prevention of Aspergillus spp. associated infections. (strong recommendation, moderate-quality evidence)4,250,251.
205. The consensus recommends for the high-risk severely immunocompromised patient, during their hospital stay when a PES is not available, their admission to a private room away from areas of construction and/or renovation with the prohibition of keeping plants or cut flowers in the room. (strong recommendation, moderate-qualityevidence)4,251,260.
206. It is recommended that hematology and/or organ transplant units establish surveillance protocols for tracking fungal infections. It is considered that an increase in cases of IFI in patients with moderate to low risk of invasive infection should encourage the investigation of the possible hospital source. (strong recommendation, moderate-quality evidence)4,250.
207. The consensus recommends the implementation of surveillance strategies to detect the increase of cases in a specific area and/or the characterization of fungal outbreaks through air quality sampling and microbiological tracing during construction and/or demolition work in or near protected areas of the hospital. (strong recommendation, high-quality evidence)251,265.
208. It is recommended that high-risk severely immunocompromised patient, and their close relatives receive specific training on prevention and control of filamentous fungal infections in order to minimize the risk of developing an IFI during hospitalization and after discharge. (strong recommendation, high-quality evidence)19,21,30,254,259-26,263,264.
209. In the immunocompromised high-risk outpatient, specific training is recommended to minimize environmental exposure to filamentous fungal conidia, such as: (a) avoiding areas with structural work, yard work, excessive dust, public restrooms and swimming pools, (b) increasing hygiene measures at home, limiting contact with pets, avoiding contact with ornamental plants and fluffy toys, and (c) avoiding ingestion of certain foods (such as unpasteurized dairy products, cheeses made from mold cultures, uncooked meat and fish or eggs, tofu, unwashed vegetables and fruits, pepper and other spices, nuts and seeds). (strong recommendation, moderate-qualityevidence)4,21,260.
2. For the implementation of antifungal stewardship (AFS) program, what are the strategies for rationalizing the use of the antifungal treatment in the patient diagnosed with an IFI/IA?
Recommendation
210. The consensus recommends the implementation of an AFS to promote the appropriate use of antifungal drugs, improve the diagnosis and quality of patient care, and decrease the costs associated with the management of an IFI/IA. (strong recommendation, high-quality evidence)121,139,266-268.
211. The consensus recommends as a first step for the implementation of an AFS the creation of a multidisciplinary team with the necessary expertise for the management of IFI/IA, and including an: (a) adult and/or pediatric infectology specialist, (for the assessment of clinical signs and symptoms, diagnostic counseling, antifungal drug selection and duration of treatment), (b) hematology specialist and/or representative of the institution's clinical specialties (for risk stratification, assessment of clinical signs and symptoms and antifungal drug prescription), (c) microbiology specialist (for delivery and interpretation of diagnostic tests, antifungal sensitivity testing and drug selection), (d) hospital pharmacy specialist (for dosing, pharmacokinetic issues in specific patient populations, drug interactions, TDM and interpretation), (e) nursing professional (for management, administration, monitoring and control of drugs of choice), (f) epidemiology professional with AFS training (for program implementation, monitoring and control of IFI), and (g) IPS administrative representative (for administrative management, monitoring and control). (strong recommendation, moderate-quality evidence) (Annex 9) 121,267-271.
212. The consensus considers that for the implementation of an AFS it is required to integrate the clinical context of the patient and its associated risk factors together with the interpretation of available and/or timely access diagnostic tools (conventional, biomarkers and imaging) followed by the appropriate choice of the antifungal drug. (strong recommendation, moderate-quality evidence)120,121,123,267.
a. For the implementation of an AFS, what is the role of diagnostic tools in the patient with high suspicion of developing an IFI/IA?
Recommendation
213. In the severely immunocompromised patient with a high suspicion of developing IFI/IA, the diagnosis is considered to be based on positive direct microscopy and recovery of the etiologic agent involved from a biopsy and/or sterile body fluid from the involved site. (strong recommendation, high-quality evidence) (I Diagnosis and Follow-Up of IA/Aspergillus Disease) 21,67,120,121,153.
214. The consensus recommends in the severely immunocom-promised patient with high suspicion of developing an IFI/IA, to include the use of fungal biomarkers (AGA, BDG, and/or PCR) and imaging studies, alone and/or used in combination, to rule out invasive disease and avoid the initiation of unnecessary antifungal therapy. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [value of Ags and/or biomarkers tests]) 67,120,121,139,153,266.
215. In the high-risk patient with hematologic malignancy and/ or HSCT, with/without profound and prolonged neutropenia, who does not receive prophylaxis against filamentous fungi, the consensus recommends the implementation of a diagnosis-guided early/directed treatment strategy. (strong recommendation, moderate-quality evidence) (section: EAFT and/or DAFT of IFI/IA) (Annex 3) 32,120,121,139,266.
216. In the asymptomatic patient with hematologic malignancy and/or HSCT, who is receiving prophylaxis and/or antifungal treatment against filamentous fungi, the consensus does not recommend the use of fungal biomarkers on a routine basis as a surveillance strategy guided by diagnostic tools. (strong recommendation, moderate-quality evidence)32,121,139,153,266.
217. In the severely immunocompromised patient, such as the patient with hematologic malignancy and/or HSCT and/ or SOTR, with evidence of imaging abnormalities and negative fungal biomarkers, the consensus recommends considering taking a biopsy from the involved site and/or additional testing to perform the diagnostic approach ofan IFI/IA. (strong recommendation, high-quality evidence)32,120,121,139,153,266,272,273.
218. The consensus considers that in the patient with hematologic malignancy and/or HSCT, with/without profound and prolonged neutropenia, with a high suspicion of developing an IFI/IAI, surveillance guided by diagnostic tools should be initiated at the beginning of each high-risk period (e.g., during the first cycle of chemotherapy) and continued until the risk no longer exists. If the patient enters a subsequent high-risk period, the tool-guided surveillance strategy should be restarted again. (strong recommendation, moderate-quality evidence) (I Diagnosis and Follow-up of IA/Aspergillus Disease [value of Ags and/or biomarkers tests]) 32,120,121,139,153,266,272.
219. The consensus considers that in the severely immuno-compromised patient with a high suspicion of developing an IFI/IAI, the implementation of a diagnosis-guided early/directed treatment strategy could reduce the costs associated with hospital stay, without increasing the associated mortality rate. (strong recommendation, high-quality evidence)17,121,274-276.
b. For the implementation of an AFS, what are the interventions that improve the prescribing and use of antifungal drugs in the patient diagnosed with an IFI/IA?
Recommendation
220. The consensus recommends that the activities for the implementation of an AFS should include: (a) development (creation, adaptation or adoption) of clinical practice guidelines for each hospital institution, (b) education of prescribers, trainees and patients, (c) improvement of diagnostic strategies (use of biomarkers, molecular testing, TDM, imaging, clinical decision support systems), (d) assessment of prescriptions with preauthorization measures and/or prospective audit with feedback (indication of antifungal, prescribed dose, follow-up of dose adjustment, risk of drug-drug interactions, treatment duration and treatment adjustment according to the microbiological findings), and (e) monitoring of process and outcome indicators according to the interventions (antifungal consumption, adherence to guidelines, microbiological and clinical outcomes). (strong recommendation, moderate-quality evidence) (Annex 9) 32,121,153,267,272,277.
i. What is the recommendation for measuring antifungal drug consumption as part of the implementation of an AFS?
Recommendation
221. The consensus considers that with the implementation of an AFS, the impact on antifungal prescription and patient outcome should be evaluated in order to justify the management of ongoing resources. (strong recommendation, moderate-quality evidence)120,121,278.
222. For the implementation of an AFS, the measurement of antifungal consumption by calculation of days on therapy (DOT) and/or defined daily dose (DDD) is recommended in each hospital institution. (strong recommendation, moderate-quality evidence)279,280.














