Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Perspectivas en Nutrición Humana
Print version ISSN 0124-4108
Perspect Nut Hum vol.15 no.1 Medellín Jan./June 2013
RESEARCH
Antioxidant and antiproliferative activity of ethanolic and aqueous extracts from leaves and fruits juice Passiflora edulis
Actividad antioxidante y antiproliferativa de extractos etanólico y acuoso de las hojas y el jugo del fruto de Passiflora edulis
Johanny Aguillón Osma1; María Elena Maldonado2; Nelsy Loango Chamorro1; Sandra Sulay Arango Varela3; Patricia Landázuri1
1 Grupo de Estudio en Enfermedades Cardiovasculares y Metabólicas (GECAVYME). Facultad de Ciencias de la Salud, Universidad del Quindío. Armenia-Colombia. jaguillon@uniquindio.edu.co
2 Grupo de Investigación Impacto de los Componentes Alimentarios en la Salud (ICAS). Escuela de Nutrición y Dietética, Universidad de Antioquia. Medellín-Colombia.
3 Grupo Sinergia. Facultad de Ingeniería, Instituto Tecnológico Metropolitano. Medellín-Colombia.
Como citar este artículo: Aguillón Osma J, Maldonado ME, Loango N, Arango Varela SS, Landázuri P. Antioxidant and antiproliferative activity of ethanolic and aqueous extracts from leaves and fruits juice Passiflora edulis. Perspect Nutr Humana. 2013;15:
Artículo recibido: 12 de diciembre de 2012; Aprobado: 20 de marzo de 2013
ABSTRACT
Background: Extracts from a variety of fruit trees have been used for therapeutic applications for preventing oxidative stress associated to chronic diseases. Objective: To investigate the antioxidant and antiproliferative activity of ethanolic and aqueous extracts from leaves and fruits of Passiflora edulis. Materials and methods: A preliminary phytochemical screening was performed; antioxidant activity was evaluated through DPPH assay, the scavenging activity for hydroxyl radical, antihemolytic activity and total phenolic content; cytotoxic and antiproliferative activities were evaluated by MTT and sulforhodamine B assays respectively in colon adenocarcinoma SW480 cells and their metastatic-derived SW620 cells. Results: Phytochemical analyses revealed presence of tannins, flavonoids and cardiotonic glycosides. Ethanolic extract from leaves showed the best antioxidant activity (EC50 = 0.096 mg/ml) in the DPPH assay and the juice (EC50 = 0.022 mg/ml) for the Hydroxyl free radical-scavenging activity. All extracts inhibited more than 98% the hemolysis induced by H2O2. The aqueous extract from leaves showed the highest cytotoxic activity against SW480 and SW620 cells. Conclusions: Findings from this study suggest that P. edulis is a potential source of phytochemical compounds with antioxidant and antiproliferative properties.
Key words: Passiflora edulis, antioxidant, antiproliferative, cytotoxic.
RESUMEN
Antecedentes: extractos de varios árboles frutales se han utilizado en diferentes aplicaciones terapéuticas para prevenir el estrés oxidativo asociado a enfermedades crónicas. Objetivo: investigar la actividad antioxidante y antiproliferativa de extractos etanólico y acuosos de las hojas y del fruto de Passiflora edulis. Materiales y métodos: se realizó marcha fitoquímica preliminar, la actividad antioxidante se evaluó por DPPH, actividad de remoción de radicales hidroxilo, actividad antihemolítica y contenido fenólico total. En células de adenocarcinoma de colon SW480 y sus derivadas metastásicas SW620 se evaluó la actividad citotóxica y antiproliferativa por el método de MTT y sulforodamina B respectivamente. Resultados: el análisis fitoquímico reveló la presencia de taninos, flavonoides y glicósidos cardiotónicos. El extracto etanólico de las hojas mostró la mayor actividad antioxidante (EC50 = 0,096 mg/ml) por DPPH y el jugo (EC50 = 0,022 mg/ml) para remover el radical hidroxilo. Todos los extractos inhibieron más del 98% la hemólisis inducida por H2O2. El extracto acuoso de las hojas mostró la mayor actividad citotóxica y antiproliferativa contra células SW480 y SW620. Conclusiones: los hallazgos de este estudio sugieren que P. edulis es una fuente potencial de compuestos fitoquímicos con propiedades antioxidante y antiproliferativa.
Palabras clave: Passiflora edulis, antioxidante, antiproliferativo, citotóxico.
INTRODUCTION
Oxidative stress has been associated with cancer, among other diseases. This process is caused by an imbalance between the production of reactive oxygen species (ROS) and the availability of biological antioxidants, which leads to cell damage (1).
Phytochemicals constitute an important class of plant-derived compounds with beneficial health properties because of their antioxidant and free-radical scavenging abilities among other biological properties (2). The most abundantly occurring plant-derived antioxidants are polyphenols (3). Although scientific interest in these compounds is relatively recent, these have been used for centuries as a strategic alternative for the prevention and / or combined treatment of various diseases (4).
Antioxidants may protect against cancer through different mechanisms involving up-regulation of antioxidant enzymes, repair of nuclear DNA, and apoptosis (5). Passiflora edulis is a Brazilian native plant, known as passion fruit which belongs to Passifloraceas (6). Different parts of this plant have been used in traditional medicine for the treatment of insomnia, sedation, epilepsy, hypnotic, hypertension, diuretic, reduction of cholesterol and triglycerides, bronchitis, asthma, colds, antispasmodic, stomach pain, tetanus, boils, and intestinal tumors (7-10). Currently, plant-derived antioxidants have become an interesting focus of research, with the aim of finding compounds that provide cellular protection against oxidative stress and cancer development. Therefore, this study we investigated the antioxidant and antiproliferative activity of ethanolic and aqueous extracts from leaves and fruits of Passiflora edulis (passion fruit).
MATERIALS AND METHODS
Extracts preparation: Healthy P. edulis leaves and fruits were collected between 6:00 and 8:00 am on Mirador farm in the village of La Herradura, municipality of La Tebaida, Armenia, Quindío (4.4376° N, 75.8489° W), 1165 msnm. The specimens were identified by the Herbarium of Universidad del Quindío (collection number: 33974). Plant samples were transported to laboratory in sealed plastic bags and washed in distilled water. Leaves were dried at 40°C until they reached a constant weight and pulverized with a mill. The ethanolic extract was obtained using the leaves' powder in 96% ethanol (EtOH). The chlorophyll was separated with EtOH/water (11). The leaves aqueous extract was prepared dissolving the leaves powder in distilled water at 40°C and filtered. The resulting ethanolic and aqueous extracts were evaporated in a Heidolph ® rotary evaporator. A stock solution was prepared in 20% solution and stored at 4°C until use. To obtain the juice, fruits pulp and seeds were sieved (pore size 1 mm), the pulp was evaporated to dryness in a Heidolph ® rotary evaporator and dissolved in distilled water to obtain a stock solution of 1mg/ml stored at 4°C until use.
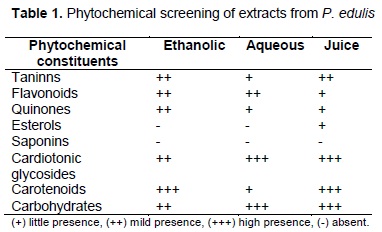
Preliminary phytochemical screening: This qualitative test was performed to identify secondary metabolites (tannins, flavonoids, quinones, sterols, saponins, cardiotonic glycosides and carotenoids) (12).
DPPH Radical scavenging activity: This assay was performed according to the method of Brand-Williams et al. (13) modified by Gunjan et al. (14). For each extract at 0.1 mg/ml; 0.2 mg/ml; 0.5 mg/ml and 1 mg/ml a DPPH solution (0.2 mM in methanol) was added (Sigma-Aldrich, United States). The mixture was incubated in darkness at 20°C for 40 min. Absorbance was measured at 517 nm using methanol as a blank. A positive control, butylated hydroxy toluene (BHT), was used in methanol at the same concentrations of the extracts. Distilled water was used as a negative control. The percentage inhibition level of DPPHby the different extracts was calculated according to the following equation: % Radical scavenging = [(AC – A)/ AC] x 100 where Acis the absorbance of the negative control and Ais the absorbance of sample (extract or BHT).
Antihemolytic activity:Was evaluated according to the procedure described by Nabavi et al. (15). Briefly, blood samples from healthy volunteer donors for hemolytic diseases were obtained by venipuncture in tubes without anticoagulant. Blood was centrifuged at 2500 rpm for 10 min, plasma and leukocytes were removed. Erythrocytes were washed three times with phosphate buffered saline (PBS) pH 7.4. A 5% (v/v) suspension of erythrocytes in PBS was added each extracts at 0.1 mg/ml, 0.2 mg/ml , 0.5 mg/ml and 1mg/ml, incubated at 37ºC for 3 hours. After centrifuge mixture reactions, supernatant was obtained and diluted in PBS. Absorbance of resulting hemoglobin in supernatants was measured at 540 nm. Erythrocytes in PBS and vitamin C were used as negative and positive controls, respectively (Sigma-Aldrich, United States). The percentage inhibition of hemolysis was calculated using the following equation: % Inhibition of hemolysis = [(Ac - A) / Ac] x 100. Where Ac is the absorbance of negative control and A is the absorbance of the sample (extract or vitamin C).
Total phenolic content: Was performed using the Folin-Ciocalteau method (14). To each extract, Folin-Ciocalteau reagent (Sigma-Aldrich, United States) and 7.5% Na2CO3 were added, mixed and incubated at 40°C for 15 minutes. The absorbance of samples was measured at 765 nm using 7.5% Na2CO3 as the blank. Data were expressed as mg gallic acid equivalents in gram of extract (mg GAE eq / g extract), according to a calibration curve.
Hydroxyl free radical-scavenging activity (HRSA): Was measured according to the method of Yang et al. (16). Briefly, to each extract at different concentrations was added 2 mM FeSO4, 6 mM salicylic acid, and 0.0 1% H2O2, incubated at 37°C for 1 hour. Ascorbic acid was used as a positive control (Sigma-Aldrich, United States). The control containing all reagents but sample was used as a blank. The HRSA was determined by measuring the absorbance at 510 nm. The percentage of HRSA was calculated with the following equation: % HRSA = [(A0 - As) / A0] x 100. Where A0 is the absorbance of negative control and As is the absorbance of samples (extract or ascorbic acid).
Total antioxidant capacity: Was determined according to the method of Prieto et al. (17). For each extract at different concentrations 0.6 M sulfuric acid, 28 mm sodium phosphate and 4 mM ammonium molybdate were added and incubated at 95°C for 90 minutes. The absorbance was measured at 695 nm against a blank. Antioxidant total capacity was expressed as mg equivalents of gram Butyl Hydroxytoluene (mg equiv/g BHT) (Sigma-Aldrich, United States), against a calibration curve.
Cell culture: SW480 and SW620 cells were obtained from the European Collection of Animal Cell Culture (ECACC, Salisbury, UK). They were cultured according to a previously described procedure (18). Briefly, cells were cultured in medium DMEM supplemented with 10% horse serum (HS), 100 U/ml penicillin, 100 μg/ml streptomycin, and 1% non-essential amino acids (Invitrogen, Cergy-Pontoise, France). For all experiments, cells were switched to assay medium containing 3% HS, and 10 μg/ml insulin, 5 μg/ml transferrin, and 5 ng/ml selenium (Invitrogen) for extracts administration 24 h after seeding.
MTT assay: Cytotoxic activity of extracts was performed in SW480 and SW620 (19). This method is based on the conversion of tetrazolium salt to formazan which is proportional to viable cells, a product generated by the activity of mitochondrial dehydrogenases. In brief, 3000 viable cells from each cell line were seeded in a 96-well cell culture plate after 24 hours, then the medium was replaced with fresh assay medium containing dilutions of extracts (0 - 400 ug/ml) dissolved in DMSO or 0.1% DMSO final concentrations, respectively for 72 h. Then, a MTT solution (5 mg/ml) was added to each well and incubated at 37°C for 4 hours in darkness. The formazan crystals were dissolved by adding acidified isopropanol (0.4 N HCl) to each well shaking continuously in darkness at room temperature (RT). Absorbance was measured at a 540 nm and 750 nm reference wavelength. The concentration able to kill 50% of cells (IC50) was calculated using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA). The absorbance of control group (non-treated cells) was considered as 100% viability. The percent inhibition was calculated using the following equation: % Inhibition = [1- (ODt/ ODc)] x 100. Where ODt is the optical density (OD) of treated cells, and ODc for control (non-treated cells).
Sulforhodamina B (SRB) assay: The effect of extracts on growth cells was studied by using the SRB assay according to Gossé et al. (20), a colorimetric assay based on staining of total cellular protein from cells with SRB dye. In brief, 3000 viable cells from each cell line were exposed to extracts for 24 hours after seeding and incubated for different times. Control cells were treated with 0.1% DMSO. Culture media were replaced every 48 hours. The cell culture was stopped by the addition of trichloroacetic acid (50% v/v), and cell proteins were determined by staining with 0.4% (w/v) SRB (Sigma-Aldrich, United States). The relationship between cell number (protein content/well) and absorbance is linear from 0 to 2x105 cells per well. All experiments were performed in triplicate.
Statistical analysis: Data were presented as mean ± standard deviation (SD) of at least three independent experiments. Linear regression analysis was used to calculate effective concentration (EC50) or IC50 and inhibition percentage correlation (dose-response). Statistical differences between groups were evaluated using ANOVA with repeated measures and p values were adjusted by Bonferroni correction (P < 0.05) using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA).
RESULTS
Preliminary phytochemical screening: Table 1 shows presence of tannins, flavonoids, quinones, carotenoids, cardiotonic glycosides and sugars in all extracts. Saponins were absent in all extracts. Sterols just were present in the juice.
DPPH Radical Scavenging Activity: Figure 1 shows the percentage of scavenging is directly proportional to the concentration of each extract (ethanolic: r2 = 0.672 (p <0.008), aqueous: r2 = 0.906 (p <0.001); juice: r2 = 0.413 (p = 0.051)). The ethanolic extract showed the strongest activity (87.4%), followed by the aqueous extract (73.4%) and the juice (15.6%), being statistically significant between them (p <0.016). No statistical difference was observed between the results obtained with the ethanol extract and positive control (p = 0.243). The EC50 for the ethanolic, aqueous and juice extracts was respectively 0.096 mg/ml, 0.448 mg/ml and 3.5 mg/ml. In addition, the ethanolic extract at 0.5 mg/ml and 1.0 mg/ml showed a DPPH value similar to the positive control (BHT) considered a highly effective antioxidant.
Antihemolytic activity: In figure 2, ethanolic and aqueous extracts shows more ability to inhibit the hemolysis H2O2-induced than positive control. The antihemolytic activity of juice was the lowest compared to the other extracts and significantly different respect to positive control (p <0.001). In addition, the antihemolytic effect using the juice was dose-dependent.
Total phenolic content: Table 2 shows that the ethanolic and aqueous extracts presented the highest concentration of phenolic compounds, 429 and 368 mg GAE/g extract respectively.
Hydroxyl free radical-scavenging activity: In figure 3 the juice showed the highest HRSA in a dose-dependent way, being the highest scavenging activity 95.7% at 1 mg/ml. Furthermore, the juice at 0.5 mg/ml and 1 mg/ml showed HRSA higher than ascorbic acid (positive control); in this study, all the extracts showed significant differences with respect to control (p = 0.015). The HRSA of aqueous extract was 82.7% at 1 mg/ml; whereas the ethanolic extract was 20.5% at 0.1 mg/ml, where the HRSA decreased as well as ethanolic extract concentration increased. The EC50 for juice was 0.22 mg/ml and for aqueous extract was 0.835 mg/ml.
Total antioxidant capacity: Results presented in table 2 show that ethanolic extract presented the highest total antioxidant activity, 1.46 and 1.36 fold increase compared to the aqueous extract and juice, respectively.
Effect of on SW480 and SW620 cell viability and cell growth: Cytotoxic and antiproliferative activities were evaluated in order to elucidate if the effect of extracts is directly correlated to the induction of cell death or to the suppression of cell proliferation. The effect of P. edulis extracts on SW480 and SW620 cells viability was investigated using MTT assay. As shown in figure 4 the viability effect on SW480 and SW620 decreased as the concentration of each extract also increased from 50 to 500 µg/ml. This effect was similar in both cell lines and no significant difference was found between treatments in each cell line compared to control (non-treated cells). The IC50 value in SW480 cells for ethanolic extract was 524 µg/ml for aqueous and 444 µg/ml for juice. In SW620 cells, IC50 value for ethanolic, aqueous extracts and juice was 442 µg/ml, 340 µg/ml and 415 µg/ml respectively.
The effect of all extracts on SW480 and SW620 cell growth is presented in figure 5, as OD at 490 nm treated or not. The OD in SW480 cell proteins (Figure 5A) was reduced by 30% and 40% with ethanolic and aqueous and juice extracts, respectively. In SW620 cells, OD was reduced by 60, 50 and 48% after treatment with aqueous, juice and ethanolic extracts, respectively. However, no statistical difference was observed between SW480 (p = 0.753) and SW620 (p = 0.795) cells treated with each extract compared to the respective non-treated cells (DMSO 0.01%).
DISCUSSION
In recent years, biomedical interest in flavonoids and tannins present in plant-derived material has increased because of their antioxidant properties applied in the prevention of certain oxidative-stress associated diseases such as cardiovascular, cancer, neurodegenerative, and diabetes. The antioxidant property of flavonoids is attributed to phenolic rings that are able to accept an electron to form relatively stable phenoxyl radicals which protects the cell from damage caused by ROS (21).
In the preliminary phytochemical screening of P. edulis extracts, the presence of tannins and flavonoids were found, which may be the compounds responsible for the antioxidant activity described here because of its well-known ability for scavenging ROS (3). The DPPH assay suggests a direct interaction between ROS and phytochemicals present in P. edulis extracts due to the high level energy and kinetic instability of ROS. This is not only attributed to the blocking action of polyphenols but by interacting with their precursors such as superoxide anion and hydrogen peroxide which lead to the hydroxyl radical synthesis which is considered the most potent oxidant.
In this study, P. edulis extracts showed an important antioxidant activity observed even at relatively low concentrations and higher than ascorbic acid, which is considered one of the best known antioxidants. The DPPH radical scavenging assay showed an important antioxidant activity of ethanolic extract (EC50: 0.096 mg/ml) indicating inhibition at at the lowest concentration (0.1 mg/ml) more than 50% of DPPH radical. This may be attributed to the presence of flavonoids and quinones in the extract that are able to transfer electrons to DPPH radical and stabilize it, consequently inhibiting their oxidant action. This EC50 value is better than one reported by Sunitha and Devaki (22) (EC50 = 0.875 mg/ml) and Vasco et al. (23) for ethanolic extract of leaves from P. edulis using the same antioxidant activity test, being the highest value of inhibition of DPPH radical 58.17% (22) and 6% at 1 mg/ml (23) indicating a low scavenging efficiency. The EC50 value closest to the result reported here for the antioxidant activity of ethanolic extract of leaves from P. edulis was presented by Rojas (21), 0.124 mg/ml. On the other hand, Ripa et al. (24) showed a higher antioxidant activity for this plant, however the solvents used to obtain the extracts are cytotoxic, this is an aspect to be considered for possible therapeutic applications; these discrepancies between the studies may be due to differences in the concentrations of the secondary metabolites in each of the extracts, originated in organic and mineral composition of the soil in which the plants were grown (25).
On other hand, phenolic compounds have also been identified as antioxidants by their ability to inhibit lipid-oxidation processes (26). In our study, the total phenolic content of the ethanolic extract (0.429 mg gallic acid/mg extract) was higher to that described by Rodriguez et al. (27) (phenolic 0.35 mg gallic acid/g extract) and Vasco et al. (23) (0.061 mg GAE/g extract) in methanolic extract indicating a low scavenging efficiency. Moreover, the correlation between the ability of each extract to inhibit DPPH radical with their respective total phenolic content was directly proportional (p = 0.009, r2 = 0.9998) and a similar finding was reported by Rudnicki et al. (28) in methanol extracts of leaves from P. edulis.
Although passion fruit juice showed DPPH and antihemolytic activities, the total phenolic content and total antioxidant capacity was the lowest compared to the other extracts. However the juice showed the highest HRSA (EC50 = 0.022 mg/ml and 95.3% inhibition) which could be attributed to the presence of sterols in juice but not in ethanolic and aqueous extracts, which have an important affinity and specificity to scavenge hydroxyl radicals compared to flavonoids and tannins (28). A similar result was reported by Murcia et al. (29) who found that the passion fruit juice inhibits 98.9% hydroxyl free radical, whereas Ferreres et al. (30) found an EC50 > 7 mg/ml value for HRSA in methanolic extract of leaves from P. edulis.
Related to the antihemolytic activity of P. edulis there are not previous reports, although some studies with extracts from Mangifera indica are able to inhibit 81.7% hemolysis (31) this was lower what was observed here with all P. edulis extracts (antihemolytic activity > 98%). Moreover, α-tochopherol was reported to inhibit hemolytic activity by 20.7% at 0.1 mg/ml (32). These results could support future pre-clinical studies that evaluate the antihemolytic activity of these extracts against cardiovascular disease, anemia or medical procedures such as hemodialysis in chronic kidney disease.
In regards to the cytotoxic and antiproliferative activities of P. edulis extracts, they presented a dose-dependent response in human colon adenocarcinoma SW480 cells and their derived metastatic SW620 cells. The highest inhibition on SW480 and SW620 cell proliferation was observed with the aqueous extract from leaves, followed by the juice and ethanolic extracts (340 – 524 mg/ml) which suggests that the extracts contain compounds that may confer antiproliferative activity. However, the National Cancer Institute (NCI) in the United States uses if an antiproliferative extract criterion of IC50 < 30 µg/ml for cancer cells (33). Using NCI criterion, this suggests that the extracts evaluated here present low activity. In accordance to our study, de Neira (34) found that different fractions of P. edulis juice (whole juice, ethanol fraction, carotenoids and polyphenols hydro soluble fraction) inhibited growth of human T cell leukemia MOLT-4 and induced cytotoxicity at 185 - 34800 µg/ml after 72 h of treatment. Also, Silva et al. (35) reported that an aqueous fraction rich in polysaccharides of P. edulis f. flavicarpa was able to inhibit by 40.6%-48.7% the proliferation of human colon adenocarcinoma cell line HCT-8 at concentrations above 100 µg/ml. Thus, taking together these data suggest that the antiproliferative and cytotoxic effect of extracts of P. edulis at concentrations greater than 100 µg/ml could be a specific characteristic of this plant.
The cytotoxic and antiproliferative activities observed in the aqueous and juice extracts might be attributed to the high presence of cardiotonic glycosides, compared to the ethanolic extract, which have showed antiproliferative and apoptotic effects on various types of cancer cells such as endometrial, ovarian (36), breast (37), leukemia (38), colon, bladder and gastric (39-40), prostate cancer, and glioblastoma (40-41). To confirm this hypothesis, further analyses are required to evaluate the anticancerigen activity of cardiotonic glycosides-enriched fractions obtained from P. edulis.
Several studies on antioxidant activity of extracts rich in polyphenols have shown protective effects against lipid peroxidation. This is of particular importance, because the oxidation of fatty acids of the membrane affects membrane proteins (enzymes, receptors) causing them to lose their functionality; lipid peroxidation appears to have an important role in the pathogenesis of various diseases, especially neurodegenerative and cardiovascular diseases (42).
In conclusion this study showed that ethanolic and aqueous extracts of P. edulis present a considerable antioxidant activity even at low concentrations which suggest that daily consumption of passion fruit could be an effective substitute for ascorbic acid and to achieve an antioxidant effect as obtained in this study. Respect to the effect on viability and cell growth on colon cancer cells, the highest activity inhibition on cell proliferation was observed using the aqueous extract and juice, which may be candidates for further analysis with polyphenols and/or cardiotonic glycosides-enriched fractions to determine their anticancer activity and useful for chemoprevention of this disease.
CONFLICTS OF INTEREST
The authors declare that they did not incur in any conflict of interest during the present study.
ACKNOWLEDGMENTS
The authors want to thank the Master course in Biomedical Science from Universidad del Quindío (Colombia), the Instituto Tecnológico Metropolitano (ITM) and Universidad de Antioquia for their financial support. In addition, special thanks to Alejandra María Giraldo for her valuable collaboration as well as to all the members from the participating laboratories..
REFERENCES
1. Dabrowska C, Moya M. Vitaminas y antioxidantes. Madrid: Sanidad y Ediciones; 2009. p.2-34. [ Links ]
2. Martínez S, González J, Culebras JM, Tuñón MJ. Los flavonoides: propiedades y acciones antioxidante: revisión. Nutr Hosp.2002;17:271-8. [ Links ]
3. Sokmen M, Angelova M, Krumova E, Pashova S, Ivancheva S, Sokmen A, et al. In vitro antioxidant activity of poliphenol extracts with antiviral properties from Geranium sanguineum L. Life Sci. 2005;76:2981-93. [ Links ]
4. Posada M, Pineda V, Agudelo G. Los antioxidantes de los alimentos y su relación con las enfermedades crónicas. Perspect Nutr Humana.2002;7:1-22. [ Links ]
5. Nijveldt R, Nood E, Hoorn D, Boelens P, Norren K, Leeuwen A. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nutr. 2001;74:418-25. [ Links ]
6. Sita SP. Morphology and pharmacology of Passiflora edulis: a review. JHerbal Med Toxicol.2009;3:1-6. [ Links ]
7. Bum E, Ngah E, Ekoundi C, Dong C, Mbomo R, Rakotonirina S, et al. Sedative and anticonvulsant properties of Passiflora edulis: dried leaves decoction in mice. Afr J Trad Compl Alternative Med.2004;1:63-71. [ Links ]
8. Kamaldeep D, Sanju D, Anupam S. Passiflora: a review update. J Ethnopharmacol. 2004;94:1-23. [ Links ]
9. Movafegh A, Alizadeh R, Hajimohamadi F, Esfehani F, Nejatfar M. Preoperative oral Passiflora reduces anxiety in ambulatory surgery patients: A double-blind, placebo-controlled study. Int Anesth Res Soc.2008;106:1728-32. [ Links ]
10. Santos KC, Tessaro SM, Davet S, Weber M, Monteiro RM, Moraes C. Sedative and anxiolytic effects of methanolic extract from the leaves of Passiflora actinia. Braz Arch Biol Technol. 2006;49:565-73. [ Links ]
11. Puricelli L, Dell I, Sartor L, Garbisa S, Caniato R. Preliminary evaluation of inhibition of matrix-metalloproteinase MMP-2 and MMP-9 by Passiflora edulis and P. foetida aqueous extracts. Fitoterapia. 2003;74:302-4. [ Links ]
12. Bilbao M. Análisis fitoquímico preliminar: química de productos naturales. Armenia: Universidad del Quindío; 1997. [ Links ]
13. Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci Technol. 1995;28:25-30. [ Links ]
14. Gunjan G, Rajkumar R, Ashok K, Lanzar M. Therapeutic potential of polar and non-polar extracts of Cyanthillium cinereum in vitro. Evid Based Compl Alternative Med. 2011;10:1-11. [ Links ]
15. Nabavi SF, Ebrahimzadeh M, Nabavi S, Eslami B. Antioxidant activity of flower, stem and leaf extracts of Ferula gummosa Boiss. Grasas Aceites. 2011;61:244-50. [ Links ]
16. Yang G, Wang D, Tang W, Chen X, Fan L, Zhang F, et al. Anti-inflammatory and antioxidant activities of Oxytropis falcate fractions and its possible anti-inflammatory mechanism. Chinese J Nat Med. 2010;8:285-92. [ Links ]
17. Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269:337-41. [ Links ]
18. Maldonado M, Bousserouel S, Gossé F, Minker C, Lobstein A, Raul F. Differential induction of apoptosis by apple procyanidins in trail-sensitive human colon tumor cells and derived trail-resistant metastatic cells. J Cancer Mol. 2009;5:21-30. [ Links ]
19. Rahman A, Choudhary MI, Thomsen WJ. Bioassay techniques for drug development. Netherlands: Harwood Academic Publishers; 2001. p.34-5. [ Links ]
20. Gossé F, Guyot S, Roussi S, Lobstein A, Fischer B, Seiler N, et al. Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis, Carcinogenesis. 2005;26:1291-5. [ Links ]
21. Rojas JP. Estudio preclínico y clínico de la seguridad y la actividad antihipertensiva de Passiflora edulis sims (maracuyá). Tesis Doctor en Farmacia y Bioquímica. Lima: Universidad Nacional de San Marcos; 2009. [ Links ]
22. Sunitha M, Devaki K. Antioxidant activity of Passiflora edulis Sims leaves. Indian J Pharm Sci. 2009;71:310-1. [ Links ]
23. Vasco C, Ruales J, Kamal-Eldin A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008;111:816-23. [ Links ]
24. Ripa F, Haque M, Nahar L, Islam M. Antibacterial, cytotoxic and antioxidant activity of Passiflora edulis Sims. Eur J Sci Res.2009;31:590-8. [ Links ]
25. García D, Ojeda F. Evaluación de los principales factores que influyen en la composición fitoquímica de Morus alba (Linn.). II polifenoles totales. Pastos Forrajes. 2004;27:59-64. [ Links ]
26. Murillo E, Lombo O, Tique M, Méndez J. Potencial antioxidante de Bauhinia kalbreyeri Harms (Fabaceae). Inform Tecnol.2007;18:65-74. [ Links ]
27. Rodríguez L, López L, García M. Determinación de la composición química y actividad antioxidante en distintos estados de madurez de frutas de consumo habitual en Colombia, mora (Rubus glaucus B), maracuyá (Passiflora edulis), guayaba (Psidium guajava L) y papayuela (Carica cundinamarcensis). Rev Asoc Col Ciencia Tecnol Alimentos. 2010;21:16-34. [ Links ]
28. Rudnicki M, Fonseca J, Dal-Pizzol F. Propiedades antioxidantes de los extractos de Passiflora alata Dryander y de Passiflora edulis Sims. Porto Alegre: Universidad Federal Do Rio Grande Do Sul; 2005. [ Links ]
29. Murcia M, Jiménez A, Martínez M. Evaluation of the antioxidant properties of mediterranean and tropical fruits compared with common food additives. J Food Protec. 2001;64:2037-46. [ Links ]
30. Ferreres F, Sousa C, Valentao P, Andrade P, Seabra R, Gil A. New C-Deoxyhexosyl flavones and antioxidant properties of Passiflora edulis leaf extract. J Agric Food Chem. 2007;55:10187-93. [ Links ]
31. Ajila C, Prasada U. Protection against hydrogen peroxide induced oxidative damage in rat erythrocytes by Mangifera indica L.peel extract. Food Chem Toxicol. 2008;46:303-9. [ Links ]
32. Zhu Q, Holt R, Lazarus S, Orozco T, Keen C. Inhibitory effects of cocoa flavanols and procyanidin oligomers on free radical induced erythrocyte hemolysis. Exp Biol Med. 2002;227:321-9. [ Links ]
33. Suffness M, Pezzuto JM. Assays related to cancer drug discovery. In: Hostettmann K, ed. Methods in plant biochemistry: assays for bioactivity. London: Academic; 1990. p.71-133. [ Links ]
34. De Neira CM. The effects of yellos passion fruit, Passiflora edulis Flavicarpa, phytochemicals on cell cycle arrest and apoptosis of leukemia lymphoma MOLT-4 cell line. Thesis for Master Science. Florida: University of Florida; 2003. [ Links ]
35. Silva D, Freitas A, Barros F, Lins K, Alves A, Alencar N, et al. Polysaccharide isolated from Passiflora edulis: Characterization and antitumor properties. Carbohydr Polymer. 2012;87:139-45. [ Links ]
36. Takai N, Ueda T, Nishida M, Nasu K, Narahara H. Bufalin induces growth inhibition, cell cycle arrest and apoptosis in human endometrial and ovarian cancer cells. Int J Mol Med. 2008;21:637-43. [ Links ]
37. Yan S, Qu X, Xu C, Zhu Z, Zhang L, Xu L, et al. Down-regulation of Cbl-b by bufalin results in up-regulation of DR4/DR5 and sensitization of TRAIL-induced apoptosis in breast cancer cells. J Cancer Res Clin Oncol. 2012;138:1279-89. [ Links ]
38. Kawazoe N, Aiuchi T, Masuda Y, Nakajo S, Nakaya K. Induction of apoptosis by bufalin in human tumor cells is associated with a change of intracellular concentration of Na+ ions. J Biochem. 1996;126:278-86. [ Links ]
39. Zhu Z, Sun H, Ma G, Wang Z, Li E, Liu Y. Bufalin induces lung cancer cell apoptosis via the inhibition of PI3K/Akt pathway. Int J Mol Sci. 2012;13:2025-35. [ Links ]
40 .Perrone A, Capasso A, Festa M, Kemertelidze E, Pizza C, Skhirtladze A, et al. Antiproliferative steroidal glycosides from Digitalis ciliata. Fitoterapia. 2012;83:554-62. [ Links ]
41. Elbaz H, Stueckle T, Wang Y, O'Doherty G, Lowry D, Sargent L, et al. Digitoxin and a synthetic monosaccharide analog inhibit cell viability in lung cancer cells. Toxicol Appl Pharmacol. 2012;258:51-60. [ Links ]
42. Rudniki M, Oliveira M, Pereira T, Regginatto F, Pizzol F, Fonseca J. Antioxidant and antiglycation properties of Passiflora edulis extracts. Food Chem. 2007;100:719-24. [ Links ]