Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista Facultad Nacional de Agronomía Medellín
Print version ISSN 0304-2847
Rev. Fac. Nac. Agron. Medellín vol.68 no.2 Medellín July/Dec. 2015
https://doi.org/10.15446/rfnam.v68n2.50987
The impact of storage conditions on the stability of sugarcane powder biofortified with kefir grains
Impacto de las condiciones de almacenamiento sobre la estabilidad del polvo de caña biofortificado con gránulos de kéfir
Blanca Cecilia Salazar Alzate1; Misael Cortés Rodríguez2 and Olga Montoya Campuzano3
1 Associate Professor. Universidad Nacional de Colombia - Sede Medellín - Facultad de Ciencias, A.A.3840, Medellín, Antioquia, Colombia. <bsalazar@unal.edu.co>
2 Titular Professor. Universidad Nacional de Colombia- Sede Medellín - Facultad de Ciencias Agrarias, A.A.1779, Medellín, Antioquia, Colombia. <mcortesro@unal.edu.co>
3 Associate Professor. Universidad Nacional de Colombia - Sede Medellín - Facultad de Ciencias, A.A.3840, Medellín, Antioquia, Colombia. <oimontoy@unal.edu.co>
Received: February 20, 2015; Accepted: April 8, 2015
DOI: http://dx.doi.org/10.15446/rfnam.v68n2.50987
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.

Abstract. The goal of this research project was to assess the quality of sugarcane powder, during storage, that had been biofortified with kefir grains (SP+KG). The powder was obtained by spray drying (SD) sugarcane concentrate that was previously fermented with kefir grains (KG). Maltodextrin, 20% w/w, was used as a drying additive, and high viability of the microbial consortium (lactobacilli, lactococci and yeast) was always sought in order to obtain a functional food enriched with probiotic microorganisms, which facilitate storage and consumption. The product was packed, both vacuum (V) and non-vacuum (WV), and stored at 10, 20 and 30°C for 180 days. The results showed significant differences (p < 0.05) in the survival rate of lactobacilli and lactococci for the time, temperature and packaging type (PT) factors; however, the yeast showed no significant differences for the PT factor. For the survival of yeast, lactococci and lactobacilli, the best temperature was 10°C and the best packaging type was V. The survival rates reached with these conditions were 62.82% (yeast), 81.22% (lactococci) and 87.82% (lactobacilli). In addition, the levels attained in terms of physical and chemical properties defined the quality specifications of the product. The sugarcane matrix is an effective vehicle of components with physiological activity such as the microorganisms present in kefir. This qualifies the product as a potential functional food.
Key words: Saccharum officinarum L., kefir, spray drying, storage.
Resumen. El objetivo de la presente investigación, fue evaluar durante el almacenamiento, la calidad del polvo de caña biofortificado con gránulos de kefir. El polvo fue obtenido por secado por aspersión del concentrado de caña panelera previa fermentación con gránulos de kefir, utilizando como aditivo de secado maltodextrina al 20% p/p, buscando siempre alta viabilidad del consorcio microbiano (lactobacilos, lactococos y levaduras), y así, obtener un alimento funcional enriquecido con microorganismos probióticos, de fácil almacenamiento y consumo. El producto, se empacó con vacío y sin vacío y se almacenó a tres temperaturas: 10, 20 y 30°C durante 180 días. Los resultados mostraron diferencias significativas (p<0,05) en el porcentaje de supervivencia de los lactobacilos y lactococos con respecto a los factores tiempo, temperatura y tipo de empacado; mientras que, las levaduras solo presentaron diferencias significativas con la temperatura y tiempo. Durante los 180 días de almacenamiento, la mejor temperatura y tipo de empacado fueron 10ºC y sin vacío, siendo la supervivencia de las levaduras, lactococos y lactobacilos de 62,82%, 81,22% y 87,82%, respectivamente; por otro lado, se alcanzaron niveles en las propiedades fisicoquímicas y físicas, que definen sus especificaciones de calidad. La matriz de caña panelera, representa un vehículo efectivo de componentes con activad fisiológica, como son los microorganismos del kefir, identificándolo como un potencial alimento funcional.
Palabras claves: Saccharum officinarum L., kefir, secado por aspersión, almacenamiento.
The consumption of probiotic microorganisms mostly occurs through the intake of foods to which they have been added. Dairy products are one of the most common substrates used for this purpose. The incorporation is achieved by fermentation (De Souza et al., 2011; Ranadheera et al., 2012; Wang et al., 2012). In addition, the market for functional foods, particularly for probiotics, has been growing during recent years and this trend is expected to continue for the next three years (BCC Research, 2014).
Probiotic microorganisms can be incorporated into foods through different methods. Among these, encapsulation with spray drying has gained interest due to its inexpensiveness and effectiveness in increasing product stability by reducing reactions between the food components (Arslan et al., 2015). Another reason for its increased popularity is that it also extends the viability of microorganisms by protecting them from moisture, oxygen and temperature (De Vos et al., 2010; Peighambardoust et al., 2011; Manojlovic' et al., 2010; Martín et al., 2015).
Studies on the encapsulation of previously isolated microorganisms involve: Lactobacillus paracasei, Lb. acidophilus, Lb. rhamnosus, Lb. plantarum, Lb. kefir, Lb. casei (Semyonov et al., 2010; Yonekura et al., 2014; Rajam and Anandharamakrishnan, 2015; Anekella and Orsat, 2013; Golowczyc et al., 2011; Paéz et al., 2012), and Lactococcus lactis (To and Etzel, 1997). Yeasts, such as Saccharomyces cerevisiae (Golowczyc et al., 2010), have also been studied.
Besides the stability seen with encapsulation, the study on the stability and survival of probiotic microorganisms in food during storage is the most important criterion when defining the shelf-life. This is how Ananta et al. (2005) studied the stability of a sample of Lactobacillus rhamnosus GG during a storage period of seven weeks at room temperature. These microorganisms were first inoculated into reconstituted skimmed milk and, then, SD was used. Similarly, Dianawati et al. (2013) studied the stability of Lactobacillus acidophilus and Lactococcus lactis ssp. cremoris during a storage period of ten weeks. These microorganisms were incorporated into a vegetable oil emulsion with protein and carbohydrates; later, spray drying was used. In other studies, such as those conducted by Ghandi et al. (2013), Lactococcus lactis ssp. cremoris was inoculated into milk lactose and whey protein; then, it was spray dried and stored at three different temperatures for 90 days. In another study, Salar-Behzadi et al. (2013) concluded that microorganism survival is affected by temperature, water activity, oxidative stress and the support material that was used.
Depending on the composition and state of the material in the food, it may undergo phase transitions of the second order during the storage period. Thus, the food passes from the glassy state to the gummy state, where its properties experience substantial transformations due to increased water mobility in the food substrate (Fazaeli et al., 2012; Masters, 1985; Bormann et al., 2013; Goula and Adamopoulos, 2010).
Most studies have been conducted with microorganisms that were isolated from different sources, including kefir grains (KG), which were then inoculated into milk and spray dried. However, there are no studies on the stability and survival rate of probiotic microorganisms that are incorporated together with the KG into a food that is not milk and then spray dried. The aim of this study was to assess the influence of storage conditions, such as temperature (10, 20 and 30°C), packaging type (with and without vacuum) and time (0, 30, 60, 90, 120, 150 and 180 days), on the quality of SP+KG.
MATERIALS AND METHODS
The KG were supplied by home consumers from the city of Medellin and the sugarcane concentrate was supplied by a rural producer of panela located in the village of Jamundí, Municipality of Girardota, Department of Antioquia, Colombia. The rate of soluble solids content in the sugarcane concentrate was 69.9%. Also, the encapsulating material was maltodextrin (MD), with a dextrose equivalent between 18 and 20 (Bell Chem International S.A.).
The spray dryer (SD) was fed with a colloidal dispersion (30 °Brix sugarcane concentrate and KG at 6% w/w) fermented at 33.5°C/30 hours. MD was later added to obtain 50% w/w of dry solids in the SD feeding. The dispersion was homogenized in an IKA Ultra-turrax UTL 50 and spray dried using pilot equipment with a disk (Vibrasec, model PSA LAB 1.5). The operating conditions were: air inlet temperature (AIT): 125°C, air outlet temperature (AOT): 65°C and atomizer disk speed (ADS): 25000 rpm. These conditions were established after optimizing the experiments using the response surface methodology (Salazar, 2015).
The storage study was conducted based on the following independent variables: temperature (10, 20 and 30°C), time (0, 30, 60, 90, 120,150 and 180 days) and PT (V and WV). Vacuum packaging (V) was performed with a model J-V002 Nw Diamond VAC packaging machine, while packaging without vacuum (WV) was done under the local barometric pressure, which was approximately 640 mm Hg/12.38 psi. The container used was an Alico brand packaging, made of coextruded polyethylene and polyamide with aroma, gas, and water barriers. Changes in sample stability were assessed in terms of microorganism survival, moisture, water activity (aw), pH, acidity and color.
The moisture content (Xw) was determined through the thermogravimetric method; for the water activity (aw), a dew point hygrometer at 25°C with an Aqua LAB Decagon series 3TE device was used; the pH was found using a Schott CG840B potentiometer. As for acidity, it was determined through volumetric titration with NaOH using phenolphthalein as an indicator (AOAC, 2002), expressed as milliequivalents of acid per gram of product (meq acid/g). Additionally, the color was determined using a spectrophotometer (X-Rite, Model SP64), with illuminant D65 and the 10 degree observer as a reference. Finally, the reflection spectrum was used to determine the CIE-L*a*b* color coordinates (L*: lightness, a*: Green - red chromaticity and b*: yellow-blue chromaticity).
Counts of viable microorganisms were performed using the serial dilution and surface stripping method in the following selective media: MRS for lactobacilli, M17 for lactococci, both incubated at 37°C/3 days under anaerobic conditions, and YGC for yeast, which was incubated at 32°C/24 to 48 hours. The results for each microbial group in each period were recorded as log CFU/g; the survival % was calculated considering the initial count (N0) and the counts in each time period (N): survival % = log (N/N0)*100.
The statistical analysis was conducted through an ANOVA. Comparisons were made to establish the minimum statistically significant differences between the means, using Fisher's test at a level of significance of 95% (P<0.05). As for the software, the statistical analyses were done using the Statgraphics Centurion XVI.I software package. The results were expressed with the mean value ± the standard deviation. These in turn were calculated from the experimental data, performed in triplicate for each storage condition.
RESULTS AND DISCUSSION
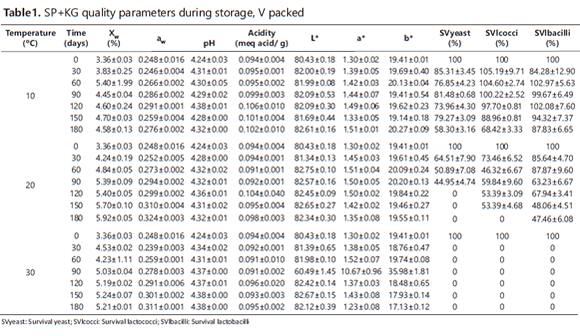
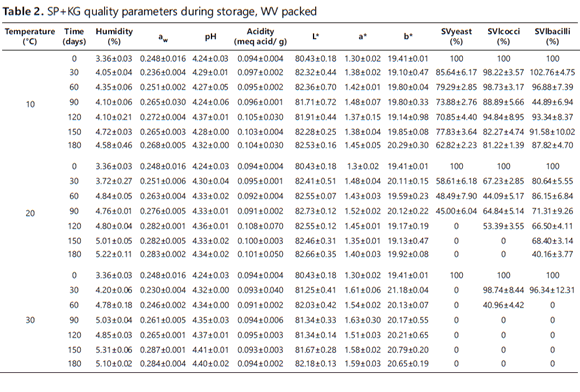
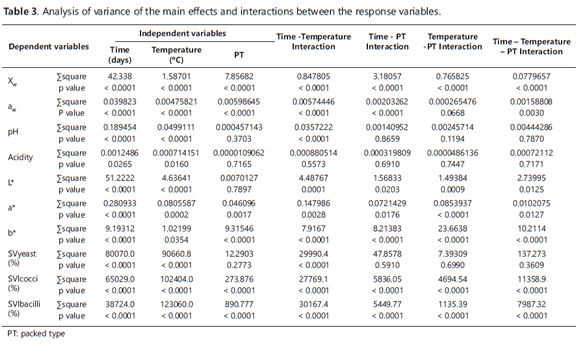
Tables 1 and 2 show the mean values plus the standard deviation for the dependent variables assessed during the SP+KG storage period. Table 1 shows the values for vacuum-packed (V) SP+KG, while Table 2 shows those of SP+KG packed without vacuum (WV). Table 3 shows the results of the analysis of variance of the main effects and interactions between the independent variables, i.e. temperature, time and PT.
The ANOVA showed significant differences (p <0.05) for Xw and aw regarding the three factors (temperature, time and PT) and their interactions, the exception being the temperature-PT interaction for aw. Additionally, it was observed that Xw and aw had a similar behavior since their values were higher as the storage time increased and lower under the low temperatures (10°C); this was due to the lower water vapor pressure to which the particles were submitted. This behavior has been reported by Teixeira et al. (1995) and Desmond et al. (2002).
At the beginning of a storage period, a product is dry and sugar-rich and has its water molecules strongly retained in the solid matrix. This results in a high absorption speed due to the difference between the surface and the headspace in terms of the chemical potential of the water. Thus, the absorption speed decreases over time due to the saturation of the active sites on the surface (Martínez et al., 1998). Furthermore, the sugars produced from sugarcane powder are very hygroscopic and, thus, have a high tendency to gain moisture from the environment (Tonon et al., 2008). This is lessened by MD because it acts as a protective agent, allowing for higher glass transition temperatures and low increases in moisture and aw during a storage period (Rodríguez-Hernández et al., 2005). In fact, Cai and Corke (2000) found hygroscopicity reduction when the MD concentration neared 20%.
The vacuum-packed samples reached higher values of Xw and aw; it is likely that the applied vacuum increased the permeability of the package coextruded polyethylene and polyamide film, thus allowing more water vapor to enter from outside. The Xw value (≅ 4.5%) obtained at 10°C for a storage period of 180 days was considered suitable for storing food powder for extended periods (Masters, 1985), similar to the findings obtained by Jayasundera et al. (2011) for fructose combined with isolated pea protein and high molecular weight surfactants. Likewise, it was within the optimal range (2.8 - 5.6%) reported by Zayed and Roos (2004) for storing Lb. salivarius subsp. salivarius inoculated into trehalose, sucrose and skimmed milk and, then, lyophilized. In general, the low values of Xw and aw obtained during the storage period (4.58 and 0.26) ensured stability against browning and hydrolytic reactions, oxidation and auto-oxidation. Similarly, these values also provided stability against enzyme activity (Singh and Heldman, 1993, cited by Marques et al., 2007). Moreover, low aw values favor the survival of lactobacilli during storage at low temperatures (Teixeira et al., 1995).
The ANOVA showed significant differences (p<0.05) for pH and acidity, particularly for the temperature and storage time factors. Conversely, there was no significant effect (P>0.05) for PT. In spite of the statistically significant differences, the variation ranges for the pH (4.24 - 4.40) and acidity (0.089 - 0.101) were very low during the storage time. This indicates that no significant acidification reactions took place in the SP+KG, which thus confirms its stability under this study's storage conditions. Such stability can be explained by the low activity of the encapsulated microorganisms and by the fact that sucrose is not hydrolyzed in its monomers due to the low aw and moisture in the product during storage.
The ANOVA showed significant differences (p<0.05) in L*, a* and b* for the temperature and time factors, as well as for all the interactions of the factors, whereas PT only influenced a* and b*. It was noted that L* had a tendency to increase mainly during the early days of the control; while parameters a* and b* showed a fluctuating behavior without setting a clear trend. In general, the variation ranges were low (L*: 80.3 -82.8; a*: 1.30-1.90 and b*: 18.7-21.2) and almost invisible to the human eye in spite of the statistical differences found. The values of the color parameters of the SP+KG identified it as a clear product (L * >>) with a creamy hue, whereas, on the a*b* Cartesian plane, the color was located in the gray area. Moreover, under the conditions of this study, the product also showed color stability versus enzymatic or chemical reactions in spite of the chemical browning reactions that might be expected due to the presence of reducing sugars (glucose and fructose) and proteins provided by the KG. Furthermore, the glassy state of the structure and the low aw values favored low molecular mobility (Roos, 1995).
The storage period began with the following counts, in log CFU / g: 5.14±0.29, 5.62±0.05 and 6.63±0.5 for yeast, lactococci and lactobacilli, respectively. The ANOVA showed significant differences (p<0.05) in the percentages for SVlbacilli and SVlcocci with respect to the three factors (temperature, time and PT) and their interactions. Likewise, the percentage for SVyeast showed significant differences with respect to the time and temperature factors and their interaction. The survival rate for the three types of microorganism behaved similarly, which decreased with the storage time. This decrease in survival was also higher as the temperature increased, the reason being that metabolism becomes weaker at low temperatures, as does diffusion and O2 partial pressure; this in turn delays the damage that may be caused to cell walls and, in general, to the macromolecules that are essential for their survival, namely: lipids and proteins, which can be oxidized or denatured (respectively) during long storage periods (Teixeira et al., 1996). Furthermore, the % of SVlcocci and % of SVlbacilli showed a greater decrease in the WV packing conditions during the first 60 days; however, their behavior was similar for both types of packaging during the remaining time.
For the three groups of microorganisms, the most favorable storage conditions during the six months were 10°C and WV since survival rates of 62.85%, 81.22% and 87.61% were reached for yeast, lactococci and lactobacilli, respectively. Golowczyc et al. (2010) obtained lower survival rates with Saccharomyces lipolytica inoculated into skimmed milk and spray-dried with an AOT of 70°C, with a storage temperature of 6°C for 150 days. During this time, the survival rate dropped to 28.57%, whereas, in our study, the survival was higher with a greater storage time and temperature. For the yeast, lactobacilli and lactococci at 30°C, the survival rate was zero after 30, 60 and 90 days, respectively. It is worth noting that, although lactococci are the microorganisms with less resistance during SD (survival: 67.14%), they showed greater resilience and lower sensitivity to the stress caused during storage (Teixeira et al., 1996).
The yeast cell wall is thin and composed of mannan oligosaccharides and β-glucans (Cepero et al., 2012). It is also susceptible to thermal damage, which makes it more sensitive to high storage temperatures.
Lactococci are Gram positive bacteria possessing thicker cell walls that are composed of peptidoglycans linked by phosphodiester bonds, which makes them more resistant (Pispa et al., 2013). Lower survival results were obtained by Ghandi et al. (2013) when they stored Lactococcus lactis ssp. cremoris for 90 days (survival rate: 53.9 % and 44.3% with and without ascorbic acid, respectively) after SD them with an AIT of 130°C and an AOT of 65°C. These microorganisms were inoculated into a protective medium composed of lactose: whey protein isolate (3:1).
Lactobacilli are Gram positive microorganisms possessing cell walls that effectively protect them against processing and storage conditions; during the SD process, this was the most tolerant group of microorganisms (85.4%). This behavior was confirmed during storage, as mentioned above. Similar results were obtained by Paéz et al. (2012) in a study in which various strains of lactobacilli stored for 75 days at 5°C reached a rate of survival of 100%. Ranadheera et al. (2015) obtained a survival rate of 93.95% with Lb. acidophilus inoculated into goat milk, which was then SD with an AIT of 195°C, an AOT of 85°C and a storage temperature of 4°C for 24 weeks. Golowczyc et al. (2010), obtained survival rates of 72% and 38.9% for Lb. plantarum and Lb. kefir in skimmed milk that was SD with an AOT of 70°C and stored at 6ºC for 150 days. However, lower survival values were obtained by Sunny-Roberts and Knorr (2009) with two strains of Lactobacillus GG (41% and 26%) inoculated into trehalose, supplemented with monosodium glutamate and SD with an AOT between 65 and 70°C and stored for four weeks.
It is possible that kefirano and the other components provided by the KG could also have had a protective effect on the microorganisms during storage. The inclusion of whole KG favored survival because the percentages obtained were high when compared with those of other studies on microorganisms isolated from kefir that were added to a food matrix and SD. This is the first report regarding the storage of food supplemented with whole KG and SD in a non-dairy matrix, which deepens the understanding of whole KG and their application to new dehydrated products.
CONCLUSIONS
Sugarcane powder was an appropriate vehicle for KG and their probiotic microorganisms.
Temperature and time were the more critical independent variables during the SP+KG storage period, as they affected the product's quality attributes, particularly the rate of survival for yeast, lactococci and lactobacilli.
Storing the SP+KG at 10°C and packing it without vacuum were the most s uitable conditions when using a container made of coextruded polyethylene and polyamide because they enabled the yeast, lactococci and lactobacilli to survive for 180 days with a survival rate of 62.82%, 81.22% and 87.82%, respectively. It is worth noting that these microbial groups have been identified as probiotics by several authors. Additionally, the product showed a low moisture content and water activity, as well as little variation in the physicochemical and physical properties, such as pH, acidity and L*, a* and b*. This indicated good stability in the food during the storage.
REFERENCES
Ananta, E., M. Volkert and D. Knorr. 2005. Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. International Dairy Journal 15(4): 399-409. En http://dx.doi.org/10.1016/j.idairyj.2004.08.004. [ Links ]
Anekella, K. and V. Orsat. 2013. Optimization of microencapsulation of probiotics in raspberry juice by spray drying. LWT - Food Science and Technology 50(1): 17-24. En http://dx.doi.org/10.1016/j.lwt.2012.08.003. [ Links ]
AOAC. 2002. Official methods of analysis of AOAC International. 17th edition. Association of Analytical Communities, Gaithersburg. [ Links ]
Arslan, S., M. Erbas, I. Tontul and A. Topuz. 2015. Microencapsulation of probiotic Saccharomyces cerevisiae var. boulardii with different wall materials by spray drying. LWT - Food Science and Technology. En: http://dx.doi.org/10.1016/j.lwt.2015.03.034. [ Links ]
BCC Research. 2014. Food And Beverage. En: Report highlights, http://www.bccresearch.com/market-research/food-and-beverage/probiotics-market-fod035d.html. consulta: Noviembre de 2014. [ Links ]
Borrmann, D., A. Rocha, S. Ferreira and M. Da Rocha. 2013. Microencapsulation of passion fruit (Passiflora) juice with n-octenylsuccinate-derivatised starch using spray-drying. Food and Bioproducts Processing 91(1): 23-27. En http://dx.doi.org/ 10.1016/j.fbp.2012.08.001. [ Links ]
Cai, Y. and H. Corke. 2000. Production and Properties of Spray-dried Amaranthus betacyanin pigments. Journal of Food Science 65(7): 1248-1252. En http://dx.doi.org/10.1111/j.1365-2621.2000.tb10273. [ Links ]
Cepero, M., S. Restrepo, A. Franco-Molano, M. Cárdenas and N. Vargas. 2012. Biología de hongos. Primera edición. Ediciones Uniandes. Bogotá [ Links ].
De Souza, R., A. Rodrigues, P. Perego, M. De Oliveira and A. Converti. 2011. Use of lactulose as prebiotic and its influence on the growth, acidification profile and viable counts of different probiotics in fermented skim milk. International journal of food microbiology 145(1): 22-27. En http://dx.doi.org/ 10.1016/j.ijfoodmicro.2010.11.011. [ Links ]
De Vos, P., M. Faas, M. Spasojevic and J. Sikkema. 2010. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. International Dairy Journal 20(4): 292-302. En http://dx.doi.org/10.1016/j.idairyj.2009.11.008. [ Links ]
Desmond, C., R. Ross, E. O'Callaghan, G. Fitzgerald and C. Stanton. 2002. Improved survival of Lactobacillus paracasei NFBC 338 in spray-dried powders containing gum acacia. Journal of Applied Microbiology 93: 1003-1011. En http://dx.doi.org/10.1046/j.1365-2672.2002.01782.x. [ Links ]
Dianawati, D., V. Mishra and N. Shah. 2013. Stability of microencapsulated Lactobacillus acidophilus and Lactococcus lactis ssp. cremoris during storage at room temperature at low aw. Food Research International 50(1): 259-265. En http://dx.doi.org/10.1016/j.foodres.2012.10.023. [ Links ]
Fazaeli, M., Z. Emam-Djomeh, A. Ashtari and M. Omid. 2012. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food and Bioproducts Processing 90(4): 667-675. En http://dx.doi.org/10.1016/j.fbp.2012.04.006. [ Links ]
Ghandi, A., I. Powell, M. Broome and B. Adhikari. 2013. Survival, fermentation activity and storage stability of spray dried Lactococcus lactis produced via different atomization regimes. Journal of Food Engineering 115(1): 83-90. En http://dx.doi.org/10.1016/j.jfoodeng.2012.09.022. [ Links ]
Golowczyc, M., J. Silva, A. Abraham, G. De Antoni and P. Teixeira. 2010. Preservation of probiotic strains isolated from kefir by spray drying. Letters in Applied Microbiology 50: 7-12. En http://dx.doi.org/10.1111/j.1472-765X.2009.02759.x. [ Links ]
Golowczyc, M., J. Silva, P. Teixeira, G. De Antoni and A. Abraham. 2011. Cellular injuries of spray-dried Lactobacillus spp. isolated from kefir and their impact on probiotic properties. International Journal of Food Microbiology 144(3): 556-560. En http://dx.doi.org/10.1016/j.ijfoodmicro.2010.11.005. [ Links ]
Goula, A. and K. Adamopoulos. 2010. A new technique for spray drying orange juice concentrate. Innovative Food Science & Emerging Technologies 11(2): 342-351. En http://dx.doi.org/10.1016/j.ifset.2009.12.001. [ Links ]
Jayasundera, M., B. Adhikari, T. Howes, and P. Aldred. 2011. Surface protein coverage and its implications on spray-drying of model sugar-rich foods: solubility, powder production and characterisation. Food Chemistry 128(4): 1003-1016. En http://dx.doi.org/10.1016/j.foodchem.2011.04.006. [ Links ]
Manojlovic', V., V. Nedovic', K. Kailasapathy and N. Zuidam. 2010. Encapsulation of Probiotics for use in food products. pp. 269-302. In: N. Zuidam, & V. Nedovic' (eds.). Encapsulation technologies for active food ingredients and food processing. Springer, Dordrecht, The Netherlands. [ Links ]
Marques, L., M. Ferreira and J. Freire. 2007. Freeze-drying of acerola (Malpighia glabra L.). Chemical Engineering and Processing: Process Intensification 46(5): 451-457. En http://dx.doi.org/10.1016/j.cep.2006.04.011. [ Links ]
Martín, M., F. Lara-Villoslada, M. Ruiz and M. Morales. 2015. Microencapsulation of bacteria: a review of different technologies and their impact on the probiotic effects. Innovative Food Science & Emerging Technologies 27: 15-25. En http://dx.doi.org/10.1016/j.ifset.2014.09.010. [ Links ]
Martínez, N., A. Andrés, A. Chiralt and P. Fito. 1998. Termodinámica y cinética de sistemas: alimento entorno. Universidad Politécnica de Valencia. [ Links ]
Masters, K. 1985. Spray Drying Handbook.Cuarta Edición. The Pitman Press, Great Britain. [ Links ]
Paéz, R., L. Lavari, G. Vinderola, G. Audero, A. Cuatrin, N. Zaritzky and others. 2012. Effect of heat treatment and spray drying on lactobacilli viability and resistance to simulated gastrointestinal digestion. Food Research International 48(2): 748-754. En http://dx.doi.org/10.1016/j.foodres.2012.06.018. [ Links ]
Peighambardoust, S., A. Golshan Tafti and J. Hesari. 2011. Application of spray drying for preservation of lactic acid starter cultures: a review. Trends in Food Science & Technology 22(5): 215-224. En http://dx.doi.org/10.1016/j.tifs.2011.01.009. [ Links ]
Pispan, S., C. Hewitt and A. Stapley. 2013. Comparison of cell survival rates of E. coli K12 and L. acidophilus undergoing spray drying. Food and Bioproducts Processing 91(4): 362-369. En http://dx.doi.org/10.1016/j.fbp.2013.01.005. [ Links ]
Rajam, R. and C. Anandharamakrishnan. 2015. Microencapsulation of Lactobacillus plantarum (MTCC 5422) with fructooligosaccharide as wall material by spray drying. LWT - Food Science and Technology 60(2): 773-780. En http://dx.doi.org/10.1016/j.lwt.2014.09.062. [ Links ]
Ranadheera, C., C. Evans, M. Adams, and S. Baines. 2015. Microencapsulation of Lactobacillus acidophilus LA-5, Bifidobacterium animalis subsp. lactis BB-12 and Propionibacterium jensenii 702 by spray drying in goat's milk. Small Ruminant Research 123: 155-159. En http://dx.doi.org/10.1016/j.smallrumres.2014.10.012. [ Links ]
Ranadheera, C., C. Evans, M. Adams and S. Baines. 2012. In vitro analysis of gastrointestinal tolerance and intestinal cell adhesion of probiotics in goat's milk ice cream and yogurt. Food Research International: 49(2): 619-625. En http://dx.doi.org/10.1016/j.foodres.2012.09.007. [ Links ]
Rodríguez-Hernández, G., R. González-García, A. Grajales-Lagunes, M. Ruiz-Cabrera and M. Abud-Archila. 2005. Spray-Drying of Cactus Pear Juice (Opuntia streptacantha): effect on the physicochemical properties of powder and reconstituted product. Drying Technology: An International Journal 23(4): 955-973. En http://dx.doi.org/10.1080/DRT-200054251. [ Links ]
Roos, Y. 1995. Phase Transitions in foods. First edition. Academic Press, Inc., Helsinki. 360 p. [ Links ]
Salar-Behzadi, S., S. Wu, S. Toegel, M. Hofrichter, I. Altenburger, F. Unger and others. 2013. Impact of heat treatment and spray drying on cellular properties and culturability of Bifidobacterium bifidum BB-12. Food Research International 54(1): 93-101. En http://dx.doi.org/10.1016/j.foodres.2013.05.024. [ Links ]
Salazar, B. 2015. Desarrollo de un alimento derivado de la caña panelera, secado por atomización y biofortificado con gránulos de kefir. Tesis Doctoral en Ciencias Agrarias. Facultad de Ciencias Agrarias. Universidad Nacional de Colombia, Medellín.106p. [ Links ]
Semyonov, D., O. Ramon, Z. Kaplun, L. Levin-Brener, N. Gurevichand E. Shimoni. 2010. Microencapsulation of Lactobacillus paracasei by spray freeze drying. Food Research International 43(1): 193-202. En http://dx.doi.org/10.1016/j.foodres.2009.09.028. [ Links ]
Sunny-Roberts, E. and D. Knorr. 2009. The protective effect of monosodium glutamate on survival of Lactobacillus rhamnosus GG and Lactobacillus rhamnosus E-97800 (E800) strains during spray-drying and storage in trehalose-containing powders. International Dairy Journal 19(4): 209-214. En http://dx.doi.org/10.1016/j.idairyj.2008.10.008. [ Links ]
Teixeira, P., H. Castro and R. Kirby. 1996. Evidence of membrane lipid oxidation of spray-dried Lactobacillus bulgaricus during storage. Letters in Applied Microbiology 22(1): 34-38. En http://dx.doi.org/10.1111/j.1472-765X.1996.tb01103.x. [ Links ]
Teixeira, P., M. Castro, F. Malcata and R. Kirby. 1995. Survival of Lactobacillus delbrueckii ssp. bulgaricus Following Spray-Drying. Journal of Dairy Science 78(5): 1025-1031. En http://dx.doi.org/10.3168/jds.S0022-0302(95)76718-2. [ Links ]
To, B. and M. Etzel. 1997. Spray drying, freeze drying, or freezing of three different lactic acid bacteria species. Journal of Food Science 62(3): 576-578. En http://dx.doi.org/10.1111/j.1365-2621.1997.tb04434.x. [ Links ]
Tonon, R., C. Brabet and M. Hubinger. 2008. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. Journal of Food Engineering 88(3): 411-418. En http://dx.doi.org/10.1016/j.jfoodeng.2008.02.029. [ Links ]
Wang, S., H. Zhu, C. Lu, Z. Kang, Y. Luo, L. Fengand X. Lu. 2012. Fermented milk supplemented with probiotics and prebiotics can effectively alter the intestinal microbiota and immunity of host animals. Journal of Dairy Science 95(9): 4813-4822. En http://dx.doi.org/10.3168/jds.2012-5426. [ Links ]
Yonekura, L., H. Sun, C. Soukoulis and I. Fisk. 2014. Microencapsulation of Lactobacillus acidophilus NCIMB 701748 in matrices containing soluble fibre by spray drying: Technological characterization, storage stability and survival after in vitro digestion. Journal of Functional Foods 6: 205-214. En http://dx.doi.org/10.1016/j.jff.2013.10.008. [ Links ]
Zayed, G. and Y. Roos. 2004. Influence of trehalose and moisture content on survival of Lactobacillus salivarius subjected to freeze-drying and storage. Process Biochemistry 39(9): 1081-1086. En http://dx.doi.org/10.1016/S0032-9592(03)00222-X. [ Links ]