Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Actualidades Biológicas
Print version ISSN 0304-3584
Actu Biol vol.30 no.89 Medellín July/Dec. 2008
HEPERTOLOGÍA
GENETIC VARIABILITY IN THE MAGDALENA RIVER TURTLE, PODOCNEMIS LEWYANA (DUMÉRIL, 1852), IN THE MOMPOS DEPRESSION, COLOMBIA
VARIABILIDAD GENÉTICA DE LA TORTUGA DEL RÍO MAGDALENA, PODOCNEMIS LEWYANA (DUMÉRIL, 1852), EN LA DEPRESIÓN MOMPOSINA, COLOMBIA
Adriana Restrepo1; Brian C. Bock2; Vivian P. Páez3
1Instituto de Biología, Universidad de Antioquia. A. A. 1226. Medellín (Antioquia), Colombia. Dirección elextrónica: <restrepoadriana78@gmail.com>
2Instituto de Biología, Universidad de Antioquia. A. A. 1226. Medellín (Antioquia), Colombia. Dirección elextrónica: <brianbock1@gmail.com>
3Instituto de Biología, Universidad de Antioquia. A. A. 1226. Medellín (Antioquia), Colombia. Dirección elextrónica: <vppaez@gmail.com>
Abstract
Two populations of the endangered Magdalena River turtle, Podocnemis lewyana, separated by 55 km in the Mompos Depression were sampled to quantify levels of genetic variation and inspect for evidence of genetic structure. Allozyme analyses resolved 22 presumptive gene loci, but only one resulted polymorphic. Genotype proportions at this locus departed from those expected under Hardy–Weinberg equilibrium, showing heterozygote deficiencies in both populations. However, allele frequencies at the locus did not differ between the two sites. The level of genetic variability documented is one of the lowest yet reported for a turtle species, and may reflect either a historical or recent bottleneck event. The lack of evidence for genetic structure suggests gene flow among the sites, at least historically, but the heterozygote deficiencies at each site also imply that inbreeding may be occurring presently within each deme.
Key words: allozymes, genetic variability, Podocnemididae, Podocnemis lewyana, population structure.
Resumen
Dos poblaciones de la tortuga amenazada, Podocnemis lewyana, en la Depresión Momposina, separadas por 55 km fueron evaluadas para cuantificar los niveles de variación genética e inspeccionar evidencias de estructura genética. Mediante un análisis de alozimas resolvimos 22 presuntivos loci genéticos, pero sólo uno resultó polimórfico. Las proporciones genotípicas en éste locus difirieron significativamente a las esperadas bajo el modelo Hardy–Weinberg, mostrando en las dos poblaciones una deficiencia de individuos heterocigotos. Además, no se encontraron diferencias en las frecuencias alélicas entre los dos sitios. El nivel de variabilidad genética documentada en este estudio es una de las más bajas reportadas para una especie de tortuga, y podría reflejar un evento de cuello de botella histórico o reciente. La falta de evidencia de estructura genética sugiere flujo genético entre los sitios, por lo menos históricamente, pero la deficiencia de individuos heterocigotos en ambos sitios podría implicar que ha ocurrido entrecruzamiento reciente dentro de cada deme.
Palabras clave: alozimas, estructura poblacional, Podocnemididae, Podocnemis lewyana, variabilidad genética.
INTRODUCTION
The Magdalena River turtle, Podocnemis lewyana bia, occurring in the Sinú, San Jorge, and Mag(Dumeril, 1852), is a biogeographic anomaly, be–dalena river drainages. The known limits to its ing the only species in the Family Podocnemididae distribution are, to the south, Tolima Department to occur to the northwest of the Andes Mountains (Prado municipality), to the north, Magdalena (Sánchez–C. et al., 1995). It is endemic to Colom–Department (Santa Marta), to the east in the upper Cesar River and Lebrija River of the Cesar Department, and to the west in the Sinú River of the Córdoba Department (Gallego–G., 2004). The species is listed in the Colombian Red Book of Reptiles as endangered (EN A1acd + 2acd; Castaño–M., 2002), but adequate conservation programs are lacking, and human consumption of eggs and adults, especially nesting females, is common practice throughout the range of the species (Restrepo et al., 2008).
The basic biology of P. lewyana is poorly known, and to date, only six studies on the species have been published (Castaño–M., 1986; Gallego–G. and Castaño–M., 2008, Hurtado, 1973; Páez et al., in press; Restrepo et al., 2008, Vargas–R. et al., 2007). Vargas–R. et al. (2007) made the first effort to genetically characterize P. lewyana by sequencing 488 bp of the cytochrome b mtDNA gene from 109 individuals obtained throughout the range of the species, including all three major drainages where it occurs. Despite this broad geographic sampling, they found only two haplotypes and no evidence of genetic structure among or within drainages. They therefore tentatively concluded that P. lewyana is genetically depauperate and spatially homogenous, but urged that this be corroborated through analyses with other molecular markers. Their results contrast with those of previous studies of the congeners P. expansa and P. unifilis that found higher levels of genetic diversity and evidence of population structure within the same drainage (Bock et al., 2001; Escalona et al., in press; Valenzuela, 2001), or at least between drainages (Pearse et al., 2006; Sites et al., 1999), using allozyme and microsatellite markers.
Knowledge of the genetic status of a population is important for its adequate conservation and management (Kohn et al., 2006). Because genetic variation may be rapidly lost due to genetic drift in small demes, its preservation is a priority for highly threatened species (Avise, 2004). This is because populations with more genetic variation, and concomitantly with higher levels of mean individual heterozygosities, will have better chances to survive over ecological or evolutionary time scales (Frankham et al., 2002). Knowledge of the levels of intra–specific genetic differentiation also is useful for making inferences concerning the micro–evolutionary processes that have influenced a species historically. Documenting the existence of genetic differences between populations at many polymorphic loci may suggest that they have been diverging randomly with restricted gene flow among them, and therefore may be considered as independent management units (Moritz, 1994a, 1994b); while differences at only one of many polymorphic loci (assuming it is a protein coding locus) may reflect adaptive divergence at that locus due to differing selective pressures at each site (Frankham et al., 2002). Given that genetic variability is the prime material upon which natural selection acts, demonstrating genetic differences between populations provides an indirect indication of the existence of current local adaptations or of the future adaptive potential of the populations (Bohonak, 1999).
In this study, we employed allozymes to quantify the levels of genetic variability within, and inspect for evidence of genetic structure between, two populations of P. lewyana separated by approximately 55 km. Our goal was to corroborate or refute the conclusions of Vargas–R. et al. (2007) concerning the low levels of genetic variation and lack of genetic structure in this species, by inspecting a far greater number of protein coding loci.
MATERIALS AND METHODS
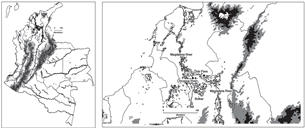
Study localities. This study was conducted in the Mompos Depression (figure 1), a wetland in the lower Magdalena River drainage. The depression is located in northern Colombia in the Bolívar and Magdalena Departments between 9° 00’ and 9° 22’ North and 74° 00’ and 74° 40’ West. During the rainy season, almost 50% of its area is flooded by overflow from the Magdalena, Cauca, and San Jorge rivers. Mean annual precipitation varies
Figure 1. Location of the two study sites in the Mompos Depression of northern Colombia
between 3,000 and 4,000 mm and mean annual temperature is 28 °C, with a mean relative humidity of 74%. This drainage originally was comprised principally of tropical moist forest (64%), with the remaining areas made up of tropical dry and tropical wet forest (Corpomojana, 2002). There are two annual dry seasons, from December to mid–April and from July to August, with the two rainy seasons occurring from mid–April to early July and late August to November (Turbay et al., 2000).
We obtained genetic samples from turtles from two locations in this region; the Chicagua River and Isla Pava. The Chicagua River (9° 07’ 52’’ N; 74° 37’ 43’’ W) is located in Bolívar Department and flows through the municipalities of Talaiga Nuevo, Mompos, and Pinillos (figure 1). This small river, with a length of 70 km and mean width of 100 m, bisects Margarita Island that is formed by the bifurcation of the Magdalena River into two major branches (DMAAC, 1995). The shoreline is dominated by cattle pasture, with few fragments of natural forest remaining. Although there are several human settlements along its shores, human densities are relatively low compared to other portions of the range of P. lewyana (Anonymous, 2001).
Isla Pava (9° 14’ 06’’ N; 74° 14’ 33’’ W) is an island located in the La Rinconada wetland that communicates via the Menchiquejo canal with the east shoreline of the Mompos Branch of the Magdalena River, in the Guamal municipality of the Magdalena Department (figure 1). The area of the island is only approximately 30 ha, while the La Rinconada wetland is one of the largest bodies of water in the region (approximately 164 km2; Díaz–G. et al., 2001). The shorelines of the island and wetland are primarily cattle pasture, with some fragments of second growth forest. There are 11 settlements located along the shores of the wetland, making it an area of relatively high human density.
Genetic samples were obtained from 372 neonate P. lewyana individuals collected from 34 nests during the course of another study on temperaturedependent sex determination in the species (Páez et al., in press). Of these, 84 neonates were obtained from 11 nests on Isla Pava during the 2005 nesting season and 288 neonates were obtained from 23 nests oviposited along the Chicagua River near the town of San Nicolás in 2006. Approximately 55 km straight–line distance separates these two sites, but fluvial routes between them are variable and considerably longer, depending on the levels of inundation at the time.
Samples of skeletal muscle, heart, and liver tissues were obtained from the neonates after they were sacrificed with injections of xilocain. The remaining carcasses were deposited in the Museo de Herpetología of the Universidad de Antioquia (MHUA) (MHUA’s vouchers 17289–17361, 17363–17364, 17366–17606, 17610–17651, 17653–17659 and 17661–17667). Tissue samples were stored at – 80 °C until prepared and analyzed in the Laboratorio de Ecología y Conservación Ambiental of the Universidad Nacional de Colombia, Sede Medellín using standard multi–pass horizontal starch gel electrophoresis techniques (Harris and Hopkinson, 1978; Murphy et al., 1996, Selander et al., 1971). Because individuals from the same nest do not represent independent samples of the allele frequencies in a population because they are either full– or half–siblings, we employed two different methods to select individuals for the analyses; one intended to detect the maximum amount of genetic variability present in each population, and the other to inspect for allele frequency differences between the two sites.
Genetic variability. For the survey of the levels of genetic variability in each population, samples from 123 individuals obtained from all 32 nests were used; 27 individuals from Isla Pava and 96 individuals from the Chicagua River site. Multipass electrophoresis was conducted on each tissue type, using different combinations of 14 buffer systems and 34 enzymatic staining conditions. The results of a previous study on P. expansa and P. unifilis (Bock et al., 2001) were used to select the initial survey conditions, but many additional conditions were inspected as well. When clear bands resulted for a particular combination of tissue/buffer system/staining condition, they were scored as presumptive genetic loci based upon the known structure and expression for each enzyme. Polymorphic loci were determined based upon the < 0.95 most common allele frequency criteria, and Percent Polymorphic Loci (P) and Mean Individual Heterozygosity (H) indices were calculated using the PopGene software package (Yeh et al., 1997).
Population structure. To inspect for allele frequency differences between the two sites, a maximum of three neonates were selected from each nest, for a total of 70 individuals (27 from Isla Pava and 43 from the Chicagua River site). This sampling scheme reduced but did not entirely eliminate the lack of independence among individuals for estimating allele frequencies in these populations. Although the occurrence of multiple paternity in P. lewyana has not yet been documented, it has been shown to be common in most of the other turtle species examined to date (FitzSimmons and Hart, 2007; Pearse and Avise, 2001; Uller and Olsson, 2008), and multiple paternity in the study clutches also would help to reduce the problem of the lack of independence among individuals used in this analysis.
Homogenates prepared from liver tissue from each individual were run on two gels using the TC8 buffer system (Murphy et al., 1996) and stained for nine loci selected from among those resolved during the initial survey. Genotype proportions at each site were compared to those expected under Hardy–Weinberg equilibrium and allele frequencies at the sites were compared using a heterogeneity X2 test, using the PopGene software package (Yeh et al., 1997).
RESULTS
The multi–pass allozyme survey resolved 22 presumptive gene loci (table 1), of which only one proved polymorphic (ACOH), with the same two alleles documented in both populations. Thus, for each population, and overall, P = 0.045. In the Isla Pava sample, H = 0.010 and in the Chicagua River
Table 1. The 22 presumptive gene loci scored in the allozyme survey of Podocnemis lewyana samples, with the optimal buffer system for resolution with liver tissue [* = names according to Murphey et al. (1996), with the respective International Union of Biochemistry Enzyme Commission numbers; ** = for the enzyme system AAT it was possible to resolve both mitocondrial and cytosolic loci (AAT–1 and AAT–2)]
site, H = 0.045. Observed genotype proportions in each population differed significantly from those expected under Hardy–Weinberg equilibrium (Isla Pava, X2 = 0.52, P = 0.02; Chicagua River, X2 = 6.20, P = 0.01), with heterozygote deficiencies at both sites. However, allele frequencies at the ACOH locus did not differ significantly between the sites (X2 = 0.294, P = 0.587).
DISCUSION
The level of genetic variability documented for P. lewyana in this study was low compared to levels reported for other turtle species using allozyme markers (Bock et al., 2001; Britten et al., 1997; Martínez et al., 2007; Phillips et al., 1996; Scribner et al., 1984, 1986; Scribner et al., 1993; Seidel et al., 1981, 1984). This could be the result of two non–mutually exclusive forms of genetic drift. First, the low diversity in P. lewyana may have resulted from a founder effect. The current distribution of the Family Podocnemididae is limited to the Orinoco, Essequibo, and Amazon drainages of South America, southeast of the Andes Mountains, plus the disjunct distribution of P. lewyana as the only species to presently occur in Andean drainages. Fossil evidence also shows that P. expansa once occurred in the upper Magdalena drainage (Galvis et al., 1997), but there is no fossil record for P. lewyana, and so it is not known whether the species originated in situ at the time of the uplift of the Cordillera Oriental of the Andes (in the Miocene; Lundberberg et al., 1986), or colonized later. However, a recent phylogeographic analysis of the Podocnemididae species that employed a calibrated molecular clock approach estimated that P. lewyana diverged approximately 15 million years ago in the middle Miocene, consistent with the vicariant origin hypothesis. If P. lewyana diverged thanks to its geographic isolation 15 million years ago, it may well have not experienced an initial founder effect, or at least the species would have had ample time for mutation to have restored normal levels of allozyme variation during the intervening period (Lande and Barrowclough, 1987).
However, more recent events affecting the Magdalena drainage, such as changes to its hydrology during Pleistocene, also might have produced a bottleneck in P. lewyana. There is evidence that the climate in the Magdalena River valley, already under the influence of the rain shadow resulting from the uplift of the Cordillera Oriental and Serranía of Perijá, became even more arid during times of glacial maximums, producing marked reductions in the amount of water present in the drainage (Galvis et al., 1997), presumable translating into declines in the population sizes of its aquatic species, especially the larger ones. Consistent with this scenario, two other aquatic vertebrate species of the Magdalena River drainage, a catfish (Gallo and Díaz–S., 2003) and another freshwater turtle species (Martinez et al., 2007), also have been shown to exhibit atypically low levels of allozyme variability.
Finally, it also is possible that P. lewyana experienced a much more recent genetic bottleneck thanks to the over–exploitation of the species since its range was settled by humans (DGE, 2002; Fals, 2002). Recent surveys have shown present day P. lewyana densities are far below anecdotally reported historical levels (Gallego–G. and Castaño–M., 2008; Restrepo et al., 2008). Other species that have experienced bottlenecks in historic times also exhibit low levels of genetic variation despite high levels of gene flow during the re–expansion of their ranges (Bonnel and Selander, 1974; Larson et al., 2002).
Whatever its cause, our results are consistent with those of Vargas–R. et al. (2007) in showing P. lewyana to be genetically depauperate. While low levels of variation in an mtDNA marker could be an artifact of a selective sweep (Bazin et al., 2006; Maynard–Smith and Haigh, 1974), multilocus allozyme studies are not expected to be significantly affected by this phenomenon. In this study, 22 independent assessments concurred with the mtDNA data in showing extremely low levels of genetic variation in this species, a result with important conservation and management implications.
The results of our study also were consistent with those of Vargas–R. et al. (2007) in failing to demonstrate evidence of population structure in P. lewyana, although the statistical power of this conclusion is weaker, being based on evidence from only two loci (cytochrome b and ACOH). At least on the micro–geographic scale of the present study, it is plausible that the two sites have experienced gene flow historically. P. lewyana is a large, long–lived species and the La Rinconada wetland is connected to the Magdalena River during periods of flooding, making aquatic movement between the two study sites feasible. The extent of terrestrial movements by presumably aquatic turtles also is often under–estimated (Gibbons, 1970, 2003). Martínez et al. (2007) recently concluded that significant gene flow has occurred among sites in the Mompos Depression in another freshwater turtle species (Trachemys callirostris).
However, this does not mean that gene flow between these two P. lewyana populations necessarily occurs at present. The fact that both populations exhibited significant heterozygote deficiencies suggests that the turtles may be inbreeding in isolated, over–exploited demes. Of course, this result also could have been an artifact of our including siblings or half–siblings in the analyses.
Thus, we feel that it is premature to propose management practices such as transferring individuals between populations to reduce inbreeding and drift. The current practice of management authorities of releasing turtles they occasionally confisca–te from poachers or venders into natural habitat without knowing their sites of origin could be producing a genetic contamination of separate stocks with their own local adaptations (Lande, 1999). The apparent lack of genetic structure and possible non–random breeding in these P. lewyana populations are tentative conclusions that need to be corroborated with other more variable co–dominant markers such as microsatellites. However, it appears that the low level of genetic variability in P. lewyana is real and not an artifact of the particular molecular marker used.
ACKNOWLEDGMENTS
This project was conducted thanks to funding support from the Turtle Conservation Fund, the Universidad de Antioquia, and the Grupo Herpetológico de Antioquia. AR thanks Cata López and her family, especially uncle Oscar and Luky. Thanks also to Juana C. Correa and Amalia Cano for their efforts in the feld and to Alejandra Cadavid and Alejandro Valencia for their assistance in the laboratory. We want to recognize the kindness of the people from Isla Pava and La Coqueta along the Chicagua River for letting us use their houses as feld stations and their invaluable help with locating nests. This study obtained permits No. 056202 and No. 0563253 from the Corporación Autónoma Regional del Sur de Bolívar (CSB), municipio de Magangué, Bolívar, Colombia.
REFERENCES
1. Anonymous. 2001. Estudio ambiental de la cuenca Magdalena–Cauca. Informe final. Convenio 003 de 1999. Instituto de Hidrología, Meteorología y Estudios Ambientales (IDEAM) y Corporación Autónoma Regional del Río Grande de la Magdalena (CorMagdalena). Bogotá D.C., Colombia. [ Links ]
2. Avise JC. 2004. Molecular markers, natural history, and evolution. Second edition. SinauerAssociates. Sunderland, Massachusetts, USA. [ Links ]
3. Bazin R, Glemin S, Galtier N. 2006. Population size does not influence mitochondrial genetic diversity in animals. Science, 312:570–572. [ Links ]
4. Bock BC, Páez VP, White MM. 2001. Genetic population structure of two endangered South American river turtle species (Podocnemis expansa and P. unifilis). Chelonian Conservation and Biology, 4:47–52. [ Links ]
5. Bohonak AJ. 1999. Dispersal, gene flow and population structure. Quarterly Review of Biology, 74:21–45. [ Links ]
6. Bonnel ML, Selander RK. 1974. Elephant seals: Genetic variation and near extinction. Science, 184:908–909. [ Links ]
7. Britten HB, Riddle BR, Brussard PF, Marlow RW, Lee TE. 1997. Genetic delineation of management units for the desert tortoise, Gopherus agassizii, in northeastern Mojave Desert. Copeia, 1997:523–530. [ Links ]
8. Castaño–M OV. 1986. Contribución al conocimiento de la reproducción de Podocnemis lewyana, Dumeril 1852 (Reptilia: Quelonia: Pelomedusidae). Caldasia, 15:71–75. [ Links ]
9.Castaño–M OV. 2002. Libro rojo de reptiles de Colombia. Instituto de Ciencias Naturales–Universidad Nacional de Colombia, Ministerio de Medio Ambiente, Conservación Internacional. Bogotá D.C. Colombia. [ Links ]
10. Corpomojana (Corporación para el Desarrollo Sostenible de la Mojana y el San Jorge). 2002. Plan de manejo ambiental de los humedales asociados al bajo Río San Jorge en los municipios de Caimito, San Benito Abad, y San Marcos, Sucre. Ministerio de Medio Ambiente y Corpomojana. San Marcos, Sucre, Colombia. [ Links ]
11. DGE (Dirección General de Ecosistemas). 2002. Programa nacional para la conservación de las tortugas marinas y continentales en Colombia. Dirección General de Ecosistemas. Bogotá, Colombia. [ Links ]
12. Díaz–G M, Camacho LA, Maestre A. 2001. Modelación de balances hídricos de ciénagas fluviales y costeras colombianas. Revista de Ingeniería, Universidad de los Andes, 13:12–30. [ Links ]
13. DMAAC (Defense Mapping Agency Aerospace Center). 1995. Riverine route maps. Maps 105–108. Defense Mapping Agency Aerospace Center. St. Louis, Missouri, USA. [ Links ]
14. Escalona T, Hernández OE, Engstrom TN, Bock BC, Vogt RC, Valenzuela N. In press. Population genetics of the endangered South American freshwater turtle, Podocnemis unifilis, inferred from microsatellite DNA data. Conservation Genetics. [ Links ]
15. Fals BO. 2002. Mompox y Loba: Historia Doble de la Costa 1. El Áncora Editores. Bogotá, Colombia. [ Links ]
16. FitzSimmons NN, Hart KM. 2007. Genetic studies of freshwater turtles and tortoises: A review of the past 70 years. Chelonian Research Monographs, 4:15–46. [ Links ]
17. Frankham R, Ballou JD, Briscou DA. 2002. Introduction to Conservation Genetics. Cambridge University Press. Cambridge, UK. [ Links ]
18. Gallego–G N, Castaño–M OV. 2008. Ecology and status of the Magdalena River turtle Podocnemis lewyana a Colombian endemic. Chelonian Conservation and Biology, 7:37–44. [ Links ]
19. Gallego–G N. 2004. Anotaciones sobre la historia natural de la tortuga de río Podocnemis lewyana (Testudinata: Podocnemididae) en el Río Sinú, Córdoba, Colombia. Undergraduate thesis. Universidad Militar Nueva Granada. Bogotá, Colombia. [ Links ]
20. Gallo H, Díaz–S J. 2003. Variabilidad genética del bagre rayado Pseudoplatystoma fasciatum (Pisces: Pimelodidae) en el Río Magdalena (Colombia). Revista de la Academia Colombiana de Ciencias Exactas, Físicas y Naturales, 27:599–605. [ Links ]
21. Gálvis G, Mojica JI, Lozano C. 1997. Peces del Catatumbo. Asociación Cravo Norte. Bogotá, Colombia. [ Links ]
22. Gibbons JW. 2003. Terrestrial habitat: A vital component for herpetofauna of isolated wetlands. Wetlands, 23:630–635. [ Links ]
23. Gibbons, JW. 1970. Terrestrial activity and the population dynamics of aquatic turtles. American Midland Naturalist, 83:404–414. [ Links ]
24. Harris H, Hopkinson DA. 1978. Handbook of enzyme electrophoresis in human genetics. North–Holland Publ. Co. Amsterdam, Netherlands. [ Links ]
25. Hurtado N. 1973. Algunos aspectos bioecológicos de Podocnemis lewyana (Dumeril, 1984) (Testudinata: Pleurodira: Pelomedusidae 1830). Biol 1. Centro de Investigaciones Biológico pesqueras del Río Magdalena. La Dorada, Caldas, Colombia. [ Links ]
26. Kohn MH, Murphy WJ, Ostrander EA, Wayne RK. Genomics and conservation genetics. Trends in Ecology and Evolution, 21:629–637. [ Links ]
27. Lande R, Barrowclough GF. 1987. Effective population size, genetic variation, and their use in population management. Pp. 87–123. In: Soulé ME (ed.). Viable Populations for Conservation. Cambridge Univ. Press, Cambridge, UK. [ Links ]
28. Lande R. 1999. Extinction risks from anthropogenic, ecological, and genetic factors. Pp. 1–22. In: Landweberg LF, Dobson AP (eds.). Genetics and the extinction of species: DNA and the conservation of biodiversity. Priceton University Press. Princeton, New Jersey, USA. [ Links ]
29. Larson S, Jameson R, Etnier M, Fleming M, Bentzen P. 2002. Loss of genetic diversity in sea otters (Enhydra lutris) associated with the fur trade of the 18th and 19th centuries. Molecular Ecology, 11:1899–1903. [ Links ]
30. Lundberg J, Machado–A A, Kay RF. 1986. Miocene Characidae fishes from Colombia: Evolutionary Stasis and Extirpation. Science, 234:208–209. [ Links ]
31. Martinez LM, Bock BC, Páez VP. 2007. Population genetics of the slider turtle (Trachemys scripta callirostris) in the Mompos Depression, Colombia. Copeia, 2007:10011005. [ Links ]
32. Maynard–S J, Haigh J. 1974. The hitch–hiking effect of a favourable gene. Genetics Research, 23:23–35. [ Links ]
33. Moritz C. 1994a. Applications of mitochondrial DNAanalisis on conservation. Molecular Ecology, 3:401–411. [ Links ]
34. Moritz C. 1994b. Defining ''evolutionarily significant units'' for conservation. Trends in Ecology and Evolution, 9:373–375. [ Links ]
35. Murphy RW, Sites JW, Buth DG, Haufler CH. 1996. Proteins: isozyme electrophoresis. Pp. 51–120. In: Hillis DM, Moritz C, Mable BK (eds.). Molecular systematics. Sinauer Associates, Inc. Sunderland, Massachusetts, USA. [ Links ]
36. Páez VP, Correa JC, Cano AM. In press. A comparison of maternal and temperature effects on sex, size, and growth of hatchlings of the Magdalena River turtle (Podocnemis lewyana) incubated under field and controlled laboratory condiditions. Copeia. [ Links ]
37. Pearse DE,ArndtAD, Valenzuela N, Millar BA, Cantarelli V, Sites JW. 2006. Estimating population structure under nonequilibrium conditions in a conservation context: continent–wide population genetics of the giant Amazon river turtle, Podocnemis expansa (Chelonia; Podocnemididae). Molecular Ecology, 15:985–1006. [ Links ]
38. Pearse DE, Avise JC. 2001. Turtle mating systems: Behavior, sperm storage, and genetic paternity. Journal of Heredity, 92:206–211. [ Links ]
39. Phillips CA, Dimmick WW, Carr JL. 1996. Conservation genetics of the common snapping turtle (Chelydra serpentina). Conservation Biology, 10:397–405. [ Links ]
40. Restrepo A, Páez VP, López C, Bock BC. 2008. Distribution and status of Podocnemis lewyana in the Magdalena River drainage of Colombia. Chelonian Conservation and Biology, 7:45–51. [ Links ]
41. Sánchez–C H, Castaño–M OV, Cárdenas–A G. 1995. Diversidad de los reptiles en Colombia. Pp. 277–325. In: Rangel–C., JO. (ed.). Colombia Diversidad Biótica I. Instituto de Ciencias Naturales, Universidad Nacional de Colombia. Editorial Guadalupe. Bogotá, Colombia. [ Links ]
42. Scribner KT, Congdon JD, Chesser RK, Smith MH. 1993. Annual differences in female reproductive success affect spatial and cohort–specific genotypic heterogeneity in painted turtles. Evolution, 47:1360–1373. [ Links ]
43. Scribner KT, Evans JE, Morreale SJ, Smith MH, Gibbons JW. 1986. Genetic divergence among populations of the yellow–bellied slider turtle (Pseudemys scripta) separated by aquatic and terrestrial habitats. Copeia, 1986:691–700. [ Links ]
44. Scribner KT, Smith MH, Gibbons JW. 1984. Genetic differentiation among local populations of the yellowbellied slider turtle (Pseudemys scripta). Herpetologica, 40:382–387. [ Links ]
45. Seidel ME, Reynolds SL, Lucchino RV. 1981. Phylogenetic relationships among musk turtles (genus Sternotherus) and genic variation in Sternotherus odoratus. Herpetologica, 37:161–165. [ Links ]
46. Selander RK, Smith MN, Yang SY, Johnson WE, Gentry JY. 1971. Biochemical polymorphism and systematic Actual Biol 30 (89): 151–159, 2008 in the genus Peromyscus. I. Variation in the old field mouse (Peromyscus polionotus). Studies in Genetics, University Texas Publication, 6:49–90. [ Links ]
47. Sites JW Jr, Bickham JW, Pytel BA, Greenbaum IF, Bates BA. 1984. Biochemical characteristics and the reconstruction of turtle phylogenies: Relationships among Batagurine genera. Systematic Zoology, 33:137–158. [ Links ]
48. Sites JW, FitzSimmons NN, Da Silva JN, Canterelli VH. 1999. Conservation genetics of the giantAmazon River turtle (Podocnemis expansa; Pelomedusidae): Inferences from two classes of molecular markers. Chelonian Conservation and Biology, 3:454–463. [ Links ]
49. Turbay S, Gómez GA, López AD, Alzate C, Álvarez AJ. 2000. La fauna de la Depresión Momposina. Editorial Lealon. Medellín, Colombia. [ Links ]
50. Uller T, Olsson M. 2008. Multiple paternity in reptiles: Patterns and processes. Molecular Ecology, 17:25662580. [ Links ]
51. Valenzuela N. 2001. Genetic differentiation among nesting beaches in the highly migratory giant river turtle (Podocnemis expansa) from Colombia. Herpetologica, 57:48–57. [ Links ]
52. Vargas–R M, Castaño–M OV, Fritz U. 2008. Molecular phylogeny and divergence times of ancient South American and Malagasy river turtles (Testudines: Pleurodira: Podocnemididae). Organisms, Diversity and Evolution, 8:388–398. [ Links ]
53. Vargas–R M, Chiari Y, Castaño–M OV, Menken SBJ. 2007. Low genetic variability in the endangered Colombian endemic freshwater turtle Podocnemis lewyana (Testudines, Podocnemididae). Contributions to Zoology, 76:1–7. [ Links ]
54. Yeh, FC, Yang RC, Boyle BJ, Ye ZH, Mao JX. 1997. POPGENE, the user–friendly shareware for population genetic analysis. Molecular Biology and Biotechnology Centre, University of Alberta. Alberta, Canada. [ Links ]
Recibido: julio 2008.
Aceptado para publicación: octubre de 2008.