INTRODUCTION
The control of immature mosquitoes is considered advantageous for preventing the transmission of vector because the larvae are usually concentrated, relatively immobile, and occupy minimal habitat compared to adults (Imbahale et al., 2011). The widespread use of chemical insecticides has developed disadvantages due to their persistent nature and residues in various environments and food (Airparif, 2016). Today, to preserve the health of non-target populations, it is necessary to focus on natural compounds from plants (Habbachi et al., 2013) by exploiting their capacity to produce secondary metabolites which can be used as new bioinsecticides (Acheuk et al., 2017). Artemisia herba-alba is a silvery perennial dwarf shrub that grows in arid and semi-arid climates, and displays rapid growth under dry and hot temperatures and in muddy areas (Tilaoui et al., 2015). In Algeria, A. herba-alba represents an important fodder resource (Belhattab et al., 2014). The essential oil of this herb has antioxidant, disinfectant, antibacterial, antileishmanial, anthelmintic, nematicide, and antispasmodic properties (Abu-Darwish et al., 2015).
In Algeria, studies on the insecticidal activity of plant extracts against the mosquito larva are limited (Benhissen et al., 2018), but in recent years has started to develop through a multitude of recent works (Acheuk et al., 2017; Belhattab et al., 2014; Benhissen et al., 2018; Habbachi et al., 2013; Matoug et al., 2017; Merabti et al., 2015, 2016). Therefore, this study is oriented toward biological control by using non-polluting, natural substances that are less harmful to develop an extract that is less expensive and effective. We examined the essential oil of A. herba-alba to evaluate its toxic activities on Cx. pipiens larvae and pupae.
MATERIALS AND METHODS
Insect
Culex pipiens are holometabolous insects that pass successively through very different stages: egg, larva, pupa, then adult (imago) (Delaunay et al., 2001). Culex females lay their eggs in the form of rafts (Michaelakis et al., 2005). The cycle breaks down into two phases: an aquatic phase for the first three stages, and an aerial phase for the last stage. Under optimal conditions, the cycle lasts from 10 to 14 days (Resseguier, 2011). Cx. pipiens larvae are found in diverse urban and peri-urban environments, especially those rich in organic matter (Jiafeng et al., 2011).
Mosquito Rearing
In the laboratory, captured larvae were sorted by larval stage and then transferred to containers for rearing in cages (20 x 20 x 20 cm) at a temperature of 25 ± 2 °C and humidity of 75 ± 10%, and a scotophase of 12 hours. A mixture of biscuit and dry yeast ensured the nutrition of the larvae (Rehimi and Soltani, 2002). Only larvae that have reached the fourth stage are the subject of a reliable identification with the help of the identification software of the Culicidae of Mediterranean Africa (Brunhes et al., 1999). Adults were fed on raspberry and cotton swabs soaked in sugar water. The blood meal, essential for egg laying, was provided by the introduction of a Petri dish containing about 5 ml of horse blood mixed with the anticoagulant heparin (Couzin, 2006).
Plant material
The plant material used in this study consisted of the aerial part of A. herba-alba, determined by comparison to a sample from the New York garden herbarium; voucher number (02708325). The identification was confirmed by Mr. Brague A., Principal Forest Inspector at the National Institute of Forest Research of the province of Djelfa. The plant was harvested in May from the Medjbara (34° 30′ N, 3° 28′ E) region in Djelfa (Figure 1). After recovery of the plant, the aerial part was cleaned. Drying was carried out naturally, protected from light and humidity, at room temperature (around 24 °C) for 15 days to preserve the molecules’ integrity as much as possible.
Extraction
The essential oil was obtained after 4 main stages: hydrodistillation, liquid-liquid extraction, water elimination, and solvent elimination.
Hydrodistillation: A quantity of 50 g of the dried plant was introduced into a balloon of 1000 ml. 500 ml of distilled water was transferred and the whole is stirred. The balloon was then placed into a hydro-distillation assembly using a Clevenger type device (Clevenger, 1928) according to the recommendations of the Hellenic Pharmacopoeia (Hellenic Pharmacopoeia, 2002).
Liquid-liquid extraction: The distillate was put into a separatory funnel, the solvent added, and the funnel is closed. Vigorous stirring was practiced for a time necessary to establish a concentration equilibrium between the two phases and degassed after it was fixed on a support with the removal of the cover. Each step was collected in an appropriate container (Abe et al., 2010).
Water Removal: To remove all traces of water, the organic phase was dried by adding a few grams of anhydrous magnesium sulfate MgSO4, then filtered using filter paper (Feknous et al., 2014).
Solvent Removal: The liquid obtained in the previous step was poured into an appropriate flask, then fixed to a rotary evaporator to carry out a simple distillation under reduced pressure at a temperature of 37 °C (Mecquenem et al., 2018). The obtained oil was stored in hermetically sealed sterile glass bottles, protected from light, and stored at a temperature of 4 °C.
Extraction efficiency of essential oil
The extraction yield was calculated using the following formula (Falleh et al., 2008):
Chemical analysis
The chemical composition of the essential oil was analyzed by gas chromatography coupled with mass spectrometry (CG/MS), which allows both a qualitative and quantitative determination of the majority of compounds of the sample (2-5 µl). The essential oil was transferred to a GC vial, diluted in hexane (1-2 ml), and sealed with a high-performance septum (Delazar et al., 2004).
The identification of the constituents was carried out by coupling a Chromatograph in the gas phase of the Clarus 680 Perkin Elmer type coupled to the Clarus SQ 8 mass spectrometer. The Rtx-5MS in fused silica (30 mx 0.25 mm ID, 0.25 pm df, RESTEK, USA) was directly coupled to the mass spectrometer (Delazar et al., 2004). The carrier gas was helium (1 ml/min). The program used was 2 min isothermal at 60 °C, then 3 °C/min at 160 °C, then 6 °C/min at 240 °C for 2 min. The temperature of the injection port was 250 °C, and the detector temperature was 240 °C. The ionization of components of the sample was performed in EI mode (70 eV). The MS scan ranged from 30 to 300 amu (Delazar et al., 2004). The individual constituents were identified by comparing their mass spectra to spectra stored in the NIST/EPA/NIH mass spectral database (Version 2.0 g from May 19, 2011).
Treatment
Sensitivity tests were carried out using the recommended protocol of the World Health Organization to test the sensitivity of the larvae towards insecticides used in control campaigns (World Health Organization, 2005). This test was carried out on 2 stages, the 4th instar larvae and pupae of Cx. pipiens. Preliminary tests with different doses were carried out to select a range of concentrations before starting the toxicity test. Three dilutions of 10% = 1μl/ml, 50% = 5μl/ml and 100% = 10μl/ml were prepared from the initial extract (1% stock solution). A total of 15 individuals (larvae/pupae) were sampled using a Pasteur pipette and placed in goblets, each containing 99 ml of water. A milliliter of each solution was then added to each previously prepared goblet. The same number of individuals were placed in a control cup containing 100 ml of water. Three repetitions were performed for each dilution as well as for the control. Mortality rates were assessed after 24, 48, and 72 hours.
Statistical analysis
The mortality values obtained for the two stages in various concentrations were considered as means. These results were subjected to a probit analysis to calculate the lethal concentrations and lethal times (LC50% LC90%, LT50%, and LT90%). This analysis was performed using the IBM SPSS Statistics program23 in Windows.
RESULTS
The effect of A. herba-alba on the mortality of Cx. pipiens
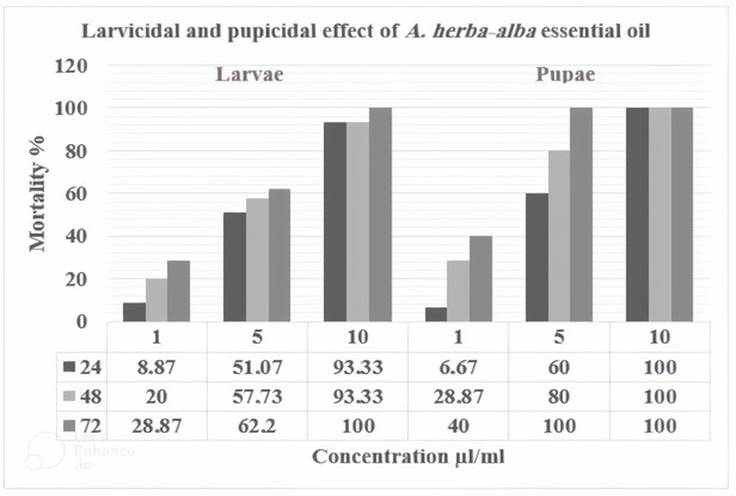
The two stages of Cx. pipiens are sensitive to A. herba-alba. This sensitivity is reflected by higher or lower mortality rates depending on the concentrations used, especially according to the time of exposure to the extract (Figure 2). In the fourth larval stage, the mortality rate ranged between 8.87% and 28.87% for the lowest concentration (1 μl/ml), while reaching 100% after 48 h when the larvae were exposed to the highest concentration (10 μl/ml). In the pupae, the mortality rate ranged between 6.67% and 40% for the lowest concentration (1 μl/ml), while reaching 100% after 72 h when pupae were exposed to the medium concentration (5 μl/ml).
Toxicological parameters of A. herba-alba
The results show a strong positive correlation between recorded mortality rates and the exposure time and/or the concentration of the extract used against mosquitoes (Tables 1 and 2). To ensure a 50% mortality of the fourth larval stage after 24 h, the concentration of A. herba-alba must be equal to 5.081 μl/ml. On the contrary, 9.128 μl/ml of A. herba-alba ensures the mortality of 90% (Table 1). After 48 h, the calculations show that the LC50% is 4.241 μl/ml, while the LC90% is 9.166 μl/ml. After 72 h of treatment, the LC50% is 3.278 μl/ml and the LC90% is 7.573 μl/ml.
On the lethal times, the concentration of 1 μl/ml of A. herba-alba eliminated 50% of the population of Cx. pipiens in 4.37 days and 90% during 7.70 days of treatment (Table 1). When 5 μl/ml of A. herba-alba extract is applied, LT50% was 0.75 days, while the LT90% was 9.77 days. To ensure a 50% mortality of the pupae after 24 h, the concentration of A. herba-alba must be equal to 4.356 μl/ml. On the contrary, 7.110 μl/ml of A. herba-alba ensures the mortality of 90% (Table 2). After 48 h, the calculations show that the LC50% is 2.579μl/ml, while the LC90% is of 6.075 μl/ml. After 72 h of treatment, the LC50% is 1.213 μl/ml and the LC90% is 2.288 μl/ml.
Table 1 Toxicological parameters of A. herba-alba essential oil in larvae treated with Cx. pipiens
| EXPOSED TIME | |||
|---|---|---|---|
| Time (hours) | 24 | 48 | 72 |
| Regression line | Y = -1.62 + 0.32x | Y = -1.1 + 0.26x | Y = -0.77 + 0.22x |
| LC 50% (µl/ml) | 5.081 | 4.241 | 3.278 |
| LC 90% (μl/ml) | 9.128 | 9.166 | 7.573 |
| 95% Confidence Interval | [0.169 0.465] | [0.126 0.394] | [0.134 0.463] |
| Chi square value | 0.055 | 0 | 0.867 |
| P value | 0.815 | 0.992 | 0.352 |
| R | 0.99 | 1 | 1 |
| CONCENTRATION USED | |||
| Concentration (μl/ml) | 1 | 5 | 10 |
| Regression line | Y = -1.71 + 0.02x | Y = -0.11 + 5.92E-3x | * |
| LT 50% (hours) | 104.910 | 18.025 | * |
| LT 90% (hours) | 184.869 | 234.389 | * |
| 95% Confidence Interval | [-0.007 0.039] | [-0.013 0.025] | [-0.022 0.056] |
| Chi square value | 0.058 | 0.004 | 0.662 |
| P value | 0.81 | 0.947 | 0.416 |
| R | 0.99 | 0.99 | * |
On the lethal times, the concentration of 1 µl of A. herba-alba eliminated 50% of the population of Cx. pipiens in the 3.3 days and 90% during 5.52 days of treatment (Table 2). When 5 μl/ml of A. herba-alba extract is applied, LT50% is 0.82 days, while the LT90% is 0.99 days.
Table 2 Toxicological parameters of A. herba-alba essential oil in pupae treated with Cx. pipiens
| EXPOSED TIME | |||
|---|---|---|---|
| Time (hours) | 24 | 48 | 72 |
| Regression line | Y = -1.94 + 0.44x | Y = -0.91 + 0.35x | * |
| LC 50% (μl/ml) | 4.356 | 2.579 | 1.213 |
| LC 90% (μl/ml) | 7.110 | 6.075 | 2.288 |
| 95% Confidence Interval | [-5.332 7.499] | [0.061 0.413] | [-11.275 13.056] |
| Chi square value | 0 | 0.49 | 0 |
| P value | 0.983 | 0.484 | 0.993 |
| R | 1 | 1 | * |
| CONCENTRATION USED | |||
| Concentration (μl/ml) | 1 | 5 | 10 |
| Regression line | Y = -2.02 + 0.03x | Y = -0.33 + 0.02x | * |
| LT50% (hours) | 79.077 | 19.693 | * |
| LT90% (hours) | 132.479 | 53.257 | * |
| 95% Confidence Interval | [0.029 0.083] | [0.009 0.067] | * |
| Chi square value | 2.848 | 0.876 | * |
| P value | 0.091 | 0.349 | * |
| R | 0.96 | 1 | * |
Average yield of AEO and its chemical characterization
The yield of A. herba-alba essential oil obtained in this study was 1.5%. Further, twenty-nine main molecules were extracted within forty minutes. We note that a large proportion was monopolized by Davanone (48.84%), which accounted for approximately half, followed by chrysanthenone (15.97%), camphor (14.84%), with the remaining proportions ranging from 0.04% to 5.69% (Table 3, Figure 3).
Table 3 Main chemical compounds (%) of A. herba-alba essential oil analyzed by the CG/SM
| Ret. Time | Compound Name | % |
|---|---|---|
| 13.604 | α-Pinene | 0.04 |
| 16.65 | Camphene | 1.34 |
| 17.575 | 2(5H)-Furanone, 5,5-dimethyl- | 0.42 |
| 9.691 | β-Myrcene | 0.16 |
| 10.031 | o-Cymene | 0.10 |
| 11.196 | Cyclohexene, 1-methyl-5-(1-methylethenyl)-, (R)- | 0.28 |
| 11.591 | Eucalyptol | 5.69 |
| 12.112 | 2(3H)-Furanone, 5-ethenyldihydro-5-methyl- | 0.21 |
| 13.647 | 1,5-Heptadien-4-ol, 3,3,6-trimethyl- | 0.20 |
| 14.743 | Bicyclo[3.1.0]hexan-3-one, 4-methyl-1-(1-methylethyl)- | 0.71 |
| 15.173 | Thujone | 0.47 |
| 15.643 | Chrysanthenone | 15.97 |
| 16.083 | Cyclohexane, 2-ethenyl-1,1-dimethyl-3-methylene- | 0.37 |
| 16.253 | Isopinocarveol | 0.70 |
| 16.568 | Camphor | 14.84 |
| 16.868 | cis-p-mentha-1(7),8-dien-2-ol | 0.63 |
| 17.329 | Pinocarvone | 0.42 |
| 17.449 | endo-Borneol | 1.61 |
| 17.899 | Terpinen-4-ol | 0.91 |
| 18.264 | Tricyclo[4.3.0.0(3,8)]nonan-2-ol,2-(aminomethyl) stereoisomer | 0.06 |
| 18.544 | α-Terpineol | 0.41 |
| 19.344 | 2-Cyclohexen-1-ol, 3-methyl-6-(1-methylethyl)-, cis- | 0.55 |
| 19.84 | Ethanol, 2-(3,3-dimethylbicyclo[2.2.1]hept-2-ylidene)- | 0.77 |
| 23.081 | Thymol | 0.19 |
| 25.612 | 3-Cyclohexene-1-methanol, α,α,4-trimethyl-, acetate | 1.51 |
| 27.728 | 3,5-Heptadienal, 2-ethylidene-6-methyl- | 1.00 |
| 28.138 | 3-Methyl-2-pent-2-enyl-cyclopent-2-enone | 0.80 |
| 35.621 | (-)-Spathulenol | 0.82 |
| 36.086 | Davanone | 48.84 |
DISCUSSION
More than 2,000 plant species with insecticidal activity have been identified (Jacobson, 1989). Some plants have evolved a wide range of physical conditions and chemical defenses against a variety of insects through substances such as phenols, polyphenols, terpenoids, and alkaloids that can be isolated using various extraction methods (Dubey, 2010).
Pranati et al. (2018) have shown the larvicidal and pupicidal effect of extracts of Clerodendrum philippinum leaves against Aedes aegypti and Anopheles stephensi with considerable mortality rates. In addition, the study by Kaura et al. (2019) reveals the larvicidal and pupicidal effect of Eucalyptus globulus essential oil, which acts quickly on Ae. aegypti and Ae. albopictus larvae and pupae, with an LC50 of 93.3 and 144.5 ppm, and an LC90 of 707.9 and 741.3 ppm, respectively.
The results of the present study reveal a considerable and variable sensitivity of Cx. pipiens to A. herba-alba essential oil, translated by rates of low to very high mortality, which correlates with the extension of time from one concentration to the other. The same observation was made by Aksorn and Mayura (2018) on the larvae of Ae. aegypti, which showed that mortality is correlated with the doses used and is increased as the exposure of larvae to insecticides is extended over time. The activity of A. herba-alba essential oil can be expressed by the diversification of the bioactive molecules which compose the essential oil. The effects of the A. herba-alba essential oil may be due to a singular action of one of the major components, dominated by Davanone (48.8%), or by a synergistic effect between several compounds towards the larvae and pupae of the mosquitos which are exposed to it.
The oil yield recorded in the present study was relatively higher than those extracted from the same species collected in Spain with 0.8% (Salido et al., 2001) and Tunisia 0.7% (Haouari and Ferchichi, 2009). While it equal to those extracted in Tunisia by Zouari et al. (2010) and by Boutemak et al. (2009) in Algeria. Also, it is lower than the one extracted in Morocco: 3.3% by Paolini et al. (2010). This difference in yield can be explained by the impact of several factors such as the nature of the species, the effect of the vegetative stage of the plant, and the edaphic conditions of the region (Ghanmi et al., 2010).
Regarding the chemical composition of this oil a variability of volatile constituents was observed in many countries from previous studies as such in Moroco [Camphor (40-70%), α-or β-Thujone (32-82% and 43-93%, respectively), Chrysanthenone (51.4%), Chrysanthenyl acetate (32-71 %), or Davanone (20-70%)] were the major components from that of Paolini et al. (2010). Whereas [Davanone (0.5-39.1%), 1,8-Cineole (0.8 -25.8%), Chrysanthenone (0.1-36.4%), Cis-Chrysanthenol (0.2-27.8%), Cis-Chrysanthenyl acetate (0.2-18.4%), p-Cymene (0.6 -20.6%), α-Pinene (0.2-17.2 %)] were reported as dominant in Spain (Salido et al., 2002). In Tunisia the major components were [Cineole (1.5-26.99%), Thujones (1-64.67%), Chrysanthenone (1-17.37%), Camphor (0.56-16.73%), Borneol (0.72-10.75%), Chrysanthenyl acetate (0.52-7.37%), Sabinyl acetate (0.53-22.46%), Davana ethers (0.65-6.23%) and Davanone (2.37-20.14%)] as referred by Haouari and Ferchichi (2009).
On the other hand, with the exception of Davanone, which is the main compound of the present work, it was not detected in the study of Abu-Darwish et al. (2015) in Jordan [β-Thujones (25.1%), α-Thujones (22.9%), Eucalyptol (20.1%) and Camphre (10%)] neither in that of Abou El-Hamd et al. (2010) in Egypt [1,8-Cineole (50%), Thujone (27%), Terpinen-4-ol (3.3%), Camphor (3%) and Borneol (3%)], as well in Iran, where Sharifian et al. (2012) reported [β-Thujone (35.66%), Camphor (34.94%), 1,8-Cineole (7.42%), α-Thujone (4.12%)] as the main components. In addition, even within Algeria, different chemical compositions of the essential oil of A. herba-alba have been recorded. For example, in the region of Djelfa, Touil and Benrebiha (2014) found Davanone (62.20%), Carvacrol (4.88%), Davana ether (3.62%), Camphor (3.48%) as major components. In Msila region, the main components announced by Dob and Benabdelkader (2006) were the Camphor (19%), trans-pinocarveol (17%), chrysanthenone (16%), b-thujone (15%), b-Thujone (32-41%), camphor (16-25 %), cineol (0.1-10%). However, Boutekedjiret et al. (1992) suggest that there is a variation of the volatile component of A. herba-alba under the seasonal change factor within the same region.
Overall, this wide chemical variability may be a result of the genetic characteristics of the plant combined with the influences of geographical locations and climatic conditions, as well as the difference in the developmental stages of the plant and the method used to obtain the essential oil (Belhattab et al., 2014; Lakehal et al., 2016). Indeed, previous studies have revealed the different bioactivities of the components of A. herba-alba extracts against many pests, such as an insecticidal activity against tobacco whitefly Bemisia tabaci (Gennadius), cotton aphid Aphis gossypii (Glover), thrips of tobacco and onion Thrips tabaci (Lindman) (Soliman, 2007). The study by Tani et al. (2008) on bean leaf beetle Acanthoscelides obtectus (Say) and of Hifnawy et al. (2001) on Cotton Worm Spodoptera littoralis (Boisduval) also revealed a toxic effect against insects. Moreover, acaricidal activity was reported against carmine spider mite Tetranychus cinnabarinus (Boisduval) per Azaizeh et al. (2007). Further Hifnawy et al. (2001) proved the ability of this essential oil to control white mice Mus musculus (Linnaeus) by provoking a rodenticidal activity.
The mechanism of action of the essential oil on insects is mainly due to neurotoxic effects involving several modes of action, including acetylcholinesterase (AChE) inhibition (Mills et al., 2004), disruption of gamma-aminobutyric acid (GABA) receptor functionality (Priestley et al., 2003) and agonist of the octopamine system (Enan, 2005).
According to Pavela (2016) the most important neurotoxic mode of action are hyperactivity followed by hyperarousal leading to rapid reversal and immobilization, as well as the insects' mouthparts becoming paralyzed which stops feeding and leads to starvation. In addition, Rattan (2010) confirms that essential oils and their constituents affect biochemical processes, which specifically disturb the endocrinological balance of insects. They can be neurotoxic or act as insect growth regulators, disrupting the normal process of morphogenesis. In insects, the result of this nerve poisoning can be immediate death or several days of paralysis before death.
In the same context, Jun-Hyung and Murray (2015) note that insecticidal activity results from a series of complex actions and contractions between a toxic tissue and an insect tissue. This mechanism of toxicity can be expressed in three steps: penetration, activation (target site interaction), and detoxification. Plant extracts act in two possible ways: a larvicidal action that can cause appreciable mortality of larvae in 1 to 12 days, or a juvenile hormone mimetic action, with an extension of the larval life span that can inhibit pupation (Rageau and Delaveau, 1979). Taking into account the toxic effect of these essential oils, a study was carried out to ensure the therapeutic safety; therefore Boukhennoufa et al. (2021) confirmed the indemnity of the toxic effect of the essential oil of A. herba-alba on the proper functioning and survival of the organism after a cutaneous exposure.
CONCLUSION
This study indicates that the essential oil of A. herba-alba has toxic properties on the larvae and pupae of Cx. pipiens. These results are encouraging and open interesting and promising horizons for its application as a bioinsecticide. These are readily available, and the cost constraint can be overcome by the low value of the LC50. However, another deep chemical study would be necessary to precisely isolate the molecule responsible for the toxic effect. In addition, a histological study is desirable to know the mode of action of this oil on the tissues of Cx. pipiens larvae and pupae.