INTRODUCTION
Dyes are organic substances that fluoresce or have an intense hue and impart color to a colorless substance or substrate by the selective absorption of light. These compounds can be classified according to their chemical structure, the application method to the fiber, or on their solubility. Among the most widely used dyes are azo dyes, which are aromatic compounds with one or more bonds (-N=N-) and represent between 65-70% of the total production of dyes. Their high demand is due to the fact that they offer a wide range of colors, present high stability and resistance to degradation by light, temperature and pH (Zuleta, 2013). The Novasyn blue light BLR compound is a diazo dye, commonly used in the textile industry to give a navy-blue coloring to materials such as cotton, leather, among others (Posada and Pulido, 2011).
It has been reported that about 15% of the dyes used in the textile industry are lost in the dyeing process and in most cases are discharged into aquatic systems without any prior treatment, generating pollution in these ecosystems (Pazarlioglu et al., 2005). Minimal amounts of dye, even in a concentration of 1 ppm, is enough to cause a negative impact because they directly affect photosynthetic processes by decreasing the penetration of solar radiation, damaging aquatic biota in general. In addition, multiple studies (Barreda et al., 2015; Chequer et al., 2009) have shown that effluents of this type have harmful effects on various organisms, which can become carcinogenic, mutagenic or teratogenic, even impacting human health (Sarmiento, 2017).
Due to the multiple disadvantages of these dyes, it has become necessary to develop effective methodologies for their removal (Martínez and Brillas, 2009; Saratale et al., 2011), which include physical methods such as adsorption, filtration, radiation and chemical methods such as the use of sodium hypochlorite or ozonation, which are expensive and cause secondary contamination (Joshi et al., 2004). In comparison, biological methods, using microorganisms such as bacteria, fungi and algae for dye removal are more attractive due to their lower costs (Gomi et al., 2011; Saratale et al., 2009).
It has been found that yeasts have a wide potential in the bioremediation of contaminated environments, because are tolerant microorganisms that exhibit a better response to extreme conditions such as the presence of heavy metals, high salts concentrations, acidic pH and high levels of xenobiotics (Al-Tohamy et al., 2020; Carballo et al., 2012; Giovanella et al., 2019), along with their ability to remediate aquatic systems with synthetic azo dyes produced by the textile industry. In this sense, Bankole et al. (2017) demonstrated that yeasts such as Diutina rugosa can be an economical, efficient, and environmentally friendly alternative in the removal of indigo-type dyes, achieving the complete removal (99.97%) of this dye (10 mg/L) in 5 days. Additionally, other yeasts such as Pichia occidentalis and Candida tropicalis have demonstrated an ability to degrade more than 98% of Acid Red B (ARB) dye in 16 h (Song et al., 2017) and to efficiently decolorize (80-90%) waters contaminated with azo dyes under aerobic conditions (Tan et al., 2013; Qu et al., 2012), respectively. Likewise, Aksu and Dönmez (2005) reported a C. tropicalis strain with the ability to remove Remazol Black B and Remazol Blue dyes (85.8 and 90.3%, respectively) within 15 days.
Considering the removal capacity of yeasts isolated from dye-contaminated sources, it is expected that industrial effluents are reservoirs of yeasts with great biotechnological potential. In this sense, the objective of this work was to isolate and characterize native yeasts isolated from wastewater contaminated with dyes and to evaluate their potential to remove the diazoic dye Novasyn blue light BLR. The most relevant result of this work was the isolation of a strain of H. opuntinae that achieved a removal of more than 90% of the dye through a biosorption mechanism, and that withstood concentrations of up to 2000 ppm without inhibitory effects on its growth. These results demonstrate that the use of yeasts in the recovery of contaminated water is an efficient biotechnological process.
MATERIALS AND METHODS
Isolation of native yeasts
Yeasts were isolated from residual waters at 2 locations near textile industry discharges in Valle de Aburrá (6°19'44.4" N, 75°33'28.8" W; 6°05'41.1" N, 75°38'14.7 " W). Samples were collected in sterile 250 ml bottles and taken to the laboratory for processing. 100 µl of each sample was seeded in YM medium (glucose 10 g/L, yeast extract 3 g/L, malt extract 3 g/L, peptone 5 g/L and agar 20 g/L) supplemented with chloramphenicol (25 ppm) and incubated at 35 °C for 72 hours. Subsequently, colonies with macro and microscopic morphological characteristics compatible with yeasts were selected. To evaluate the tolerance of the isolated colonies to the blue dye Novasyn blue light BLR, they were seeded by exhaustion on YM agar supplemented with the dye (100 ppm) and incubated at 35 °C for 72 hours.
Evaluation of dye removal ability
Yeasts with the ability to grow in the presence of the dye were evaluated in YM broth to determine their removal capacity. The percentage of removal was determined at different dye concentrations (250 and 500 ppm) and at different incubation temperatures (25 °C and 35 °C) at pH 4.0 and constant agitation (120 rpm) for 120 hours. Controls without yeast were performed to evaluate abiotic factors associated with discoloration and controls without dye to evaluate yeast viability. After 120 hours of incubation, 1 ml of the culture was taken, centrifuged at 12,000 x g for 10 min, the biomass was resuspended in sterile distilled water and its absorbance was measured at 600 nm and that of the supernatant at 560 nm and the percentage of removal was determined, calculated from the following formula (Bankole et al., 2017):
Biochemical and molecular identification
For biochemical identification, isolates with a removal rate higher than 90% were selected. From 24-hour cultures, biochemical identification was performed using the API gallery® 20 C AUX following the manufacturer's instructions (bioMérieux ®). Molecular identification was performed by sequencing of the 5.8S ITS region. DNA extraction included cell wall removal with lyticase enzyme (Sigma), cell lysis, purification with phenol:chloroform and finally precipitation with isopropanol. For amplification of the 5.8S ITS region, primers ITS1 (5`-TCC GTA GGT GAA CCT GCG G-3`) and ITS4 (5'-TCC TCC GCT GCT TAT GAA TGC-3') (White et al., 1990) were used. The PCR temperature profile was as follows: 95 °C for 10 min, followed by 30 cycles at 94 °C for 30 s, 53 °C for 30 s, 72 °C for 1 min and finally at 72 °C for 5 min. PCR products were purified and sent for sequencing at Macrogen (South Korea). The sequences were analyzed using the BLAST tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Influence of the culture medium on dye removal
To evaluate the effect of the composition of the culture medium on the percentage of removal, the isolate with the highest percentage of removal was selected. Different concentrations of glucose (2, 6 and 10 g/L), yeast extract (0, 0.1 and 0.2 g/L), ammonium sulfate (0, 0.5 and 1 g/L) and the dye Novasyn blue light BLR (250, 500 and 750 ppm) were evaluated. In addition, different pH (3, 5 and 7) were evaluated. The concentrations of magnesium sulfate and dipotassium phosphate were maintained at 0.5 g/L and 1 g/L, respectively. Cultures were incubated at 35 °C with continuous shaking (120 rpm) for 24 hours and samples were taken every 3 hours. Samples were centrifuged at 12,000 x g for 10 minutes to determine the concentration of biomass produced and the residual concentration of the dye. All assays were performed in duplicate.
Toxicity of the dye
To evaluate the inhibitory effect of dye on the growth of the yeast with the best performance in dye removal, it was incubated in YM broth with different concentrations of dye (500, 1000, 1500, 2000 ppm). The assays were performed at 35 °C under continuous agitation (120 rpm) for 24 hours. Samples were taken at the beginning and at the end of the assay. Samples were centrifuged at 12000 x g for 10 minutes to determine the biomass produced and the residual dye concentration. All assays were performed in duplicate.
Dye removal in wastewater
To evaluate the removal capacity of the selected isolate in industrial wastewater, water was collected from a textile effluent that was sterilized at 121 °C for 20 minutes and subsequently supplemented with K2 HPO4 (10 g/L), MgSO4 (0.5 g/L) and 250 ppm of the dye, the pH was adjusted to 3.0 and the culture conditions were maintained at 35 °C and continuous agitation (120 rpm) for 24 hours. Samples were taken at the beginning and end of the assay. Samples were centrifuged at 12,000 x g for 10 minutes and the percentage of discoloration was determined by measuring the absorbance of the culture supernatant at 560 nm. All assays were performed in duplicate. Additionally, spectral sweeps were performed in the 400-700 nm range, both at the beginning and at the end of each treatment in order to identify changes in the wavelength of maximum absorption of the dye.
RESULTS
Isolation and identification of yeasts with decolorizing potential
From wastewater samples collected in the Metropolitan Area of Valle de Aburrá, 22 yeast colonies with different morphological characteristics were isolated, from which 15 colonies were selected based on their ability to grow in the presence of Novasyn blue light BLR dye at a concentration of 100 ppm. The main differential macroscopic characteristic in the isolates was the coloration of the colonies, some grew as white colonies with no change in the color of the culture medium, others grew as violet colonies with discoloration of the medium (Figure 1). Biochemical and molecular identification of the strains with the highest percentage of clearance (>90%) evidenced the presence of Candida sp., Hanseniaspora sp. and Pichia sp. (Table 1).

Figure 1 Growth of yeast isolated from industrial wastewater in YM medium + Novasyn blue light BLR dye.
Table 1 Taxonomic classification of yeasts isolated from industrial wastewater and percentage of dye removal
| Isolated | ID API 20 C AUX | ID ITS 5.8S | Percent removal at 25 °C | Percent removal at 35 °C | ||
|---|---|---|---|---|---|---|
| 250 ppm | 500 ppm | 250 ppm | 500 ppm | |||
| 4 | Candida rugosa | Candida catenulata | 100 | 100 | 100 | 98.4 |
| 5 | Candida guilliermondii | Candida intermedia | 47 | 43.7 | 95.8 | 99.5 |
| 8 | Kloeckera spp. | Hanseniaspora opuntinae | 91.7 | 86.6 | 100 | 99.1 |
| 11 | Kloeckera spp. | Hanseniaspora opuntinae | 98.3 | 98.1 | 100 | 99.5 |
| 14 | Geotrichum klebahnii | Pichia kluyveri | 98.8 | 90.9 | 98.4 | 97.7 |
Dye removal test
Strains grown in the presence of the dye were subjected to a removal assay in liquid medium. Different concentrations of the dye (250 and 500 ppm) were evaluated at two incubation temperatures (25 °C and 35 °C). Most of the isolated colonies exhibited similar removal percentages at the dye concentrations evaluated, being higher than 60% at 25 °C and 35 °C (Figures 2A and 2B). The assays performed at 35 °C exhibited higher dye removal, where 9 of the 15 colonies evaluated achieved percentages higher than 90% and only two colonies presented higher removal at the lowest dye concentration. At 25 °C higher biomass production was obtained (Figures 2C and 2D). Additionally, some colonies showed an opposite behavior in dye removal, while colonies 6 and 13 achieved higher removal at 500 ppm, strains 7 and 15 showed higher removal at 250 ppm. However, no statistically significant differences in dye removal were observed at 25 and 35 °C (p > 0.05).
Influence of the culture medium on the removal of Novasyn blue light dye BLR
According to the behavior exhibited by the yeasts in the removal test, isolate 11 identified as Hanseniaspora opuntiae was selected to evaluate the effect of the composition of the culture medium on the removal. Different concentrations of glucose, yeast extract, ammonium sulfate and dye were evaluated at different pH conditions, observing that the composition of the culture medium did not influence dye removal or yeast growth, and biomass was not favored at higher concentrations of glucose or yeast extract (p > 0.05) (Figure 3A). However, dye concentration and pH were the parameters that significantly influenced removal (p < 0.05) (Figures 3B and 3C), demonstrating that an acidic environment in the medium favors dye removal at low dye concentrations (250 ppm) by H. opuntiae.
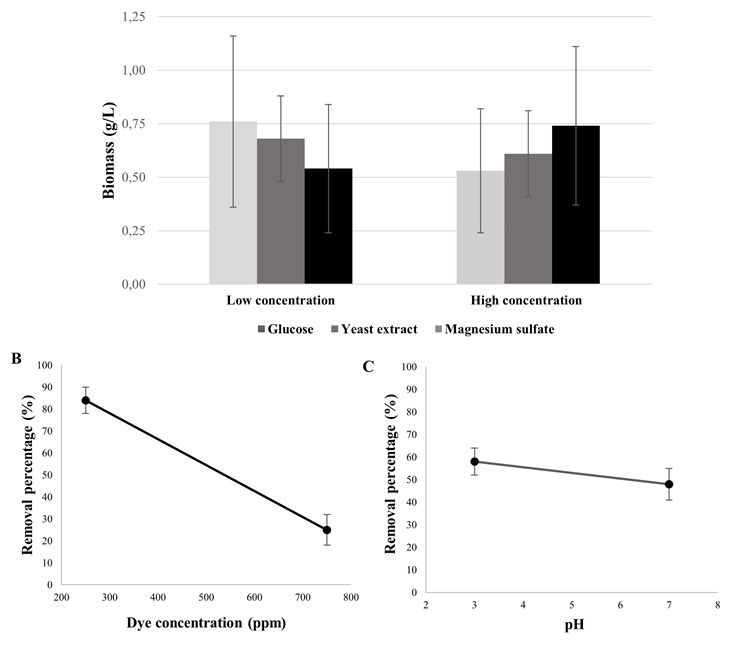
Figure 3 Effect of culture medium composition, dye concentration and pH on growth and dye removal by H. opuntinae. A. Yeast growth in synthetic medium with glucose (2, 6 and 10 g/L), yeast extract (0; 0.1 and 0.2 g/L) and ammonium sulfate (0; 0.5 and 1 g/L). B. Percent removal at 250, 500 and 750 ppm of the dye. C. Percentage of removal at pH 3, 5 and 7.
Toxicity test
To determine the effect of the dye concentration on the growth of H. opuntiae, concentrations between 500 and 2000 ppm were evaluated. The results obtained confirm that the percentage of removal and biomass formation decrease as the dye concentration increases. However, at concentrations up to 2000 ppm no inhibitory effect on the growth of H. opuntiae is observed. High concentrations of the dye may represent a stressful situation, but they are not toxic, nor do they inhibit the growth of H. opuntiae.
Wastewater removal test
To perform the textile effluent dye removal test, the culture conditions established were: dye at 250 ppm, temperature at 35 °C and pH of 3.0 with constant agitation at 120 rpm. After 24 hours of incubation, the percentage of removal was less than 30%. With respect to biomass production, it is observed that it was lower when the dye was present in the medium (0.1 g/L of biomass in the medium with dye, 0.43 g/L of biomass in the medium without dye); although in both conditions, biomass production was much lower than that obtained in the culture medium (3.96 g/L) (Figure 4A).
Finally, the absorption spectrum of the culture supernatant was evaluated in the visible light range (400-700 nm). The uninoculated colored medium presented a maximum absorbance peak at 560 nm, after the 24-hour treatment with H. opuntiae a substantial decrease in the absorbance of the medium was observed indicating decolorization, although without changes in the absorption peak or the appearance of other peaks suggesting changes in the chemical structure of the dye (Figure 4B).
DISCUSSION
Azo dyes are the most widely used in the textile industry. However, colored wastewater is difficult to treat by conventional methods and the release of these effluents into the environment has great repercussions due to their toxic, carcinogenic nature and their bioaccumulation along the trophic chain (Sathishkumar et al., 2019). Physical and chemical treatments exist to treat these effluents, but they are costly or generate toxic compounds; therefore, biological decolorization can be considered the most viable and effective method for the treatment of this kind of effluents (Jafari et al., 2013). Although the behavior of many microorganisms in dye removal has been studied, yeasts have been little explored, although some strains of Candida sp., Debaryomyces sp., Issatchenkia sp., and Magnusiomyces sp., have been reported as potential candidates for azo dye removal (Sinha et al., 2018).
In this study, the removal of the Novasyn blue light BLR compound in YM medium was higher than 60% in all the strains isolated from industrial effluents, demonstrating higher decolorization capacity at 35 °C. The positive influence of temperature on removal has been reported to be due to a decrease in the viscosity of the colored solution, increasing the rate of diffusion of dye molecules through the microbial cell wall, thus favoring its removal (Zhou et al., 2019). Studies conducted with yeasts such as Scheffersomyces spartinae and Pichia kudriavzevii demonstrated a higher decolorization capacity (98-100%) at temperatures above 30 °C (Tan et al., 2016; Roșu et al., 2018), which would be associated with the optimal yeast growth temperature and the decolorization mechanism used (Khan et al., 2012). The results obtained in this study do not demonstrate a direct relationship between biomass production and dye removal, where no significant differences in decolorization were found in cultures grown at 25 °C and 35 °C. Therefore, the mechanism employed by the yeasts isolated in this study may be associated with other factors.
The yeasts with a higher removal capacity of Novasyn blue light BLR dye were identified as Candida catenulata, C. intermedia, H. opuntiae and Pichia kluyveri. Multiple Candida sp. species have been reported in wastewater treatment of fermentative processes (Liu et al., 2019), pig farms (Martinez and Brillas, 2009) and in the decolorization of textile effluents, mainly by biosorption mechanisms (Pajot et al., 2014). Some Pichia species have demonstrated a capacity for biodegradation of azo dyes by the production of laccase-type enzymes and hydroxylation of the dye (Wang et al., 2020). For the subsequent experiments, the yeast H. opuntiae was selected considering its high percentage of removal under all conditions evaluated (25 and 35 °C, 250 and 500 ppm of dye). This yeast has been reported mainly as part of the microbiota responsible for cocoa fermentation and in the cofermentation of alcoholic beverages such as wines, beers and distillates (Figueroa et al., 2019; Gschaedler, 2017). On YM medium H. opuntiae grows as apiculate or elongated cells with bipolar budding and the colonies are creamy white to beige in color.
Biomass production has been described as an important factor in dye removal capacity with yeasts and modification of factors influencing microbial growth can affect decolorization efficiency (Zabłocka and Przystaś, 2020). In a study conducted with C. tropicalis, a direct relationship between biomass growth and decolorization capacity was determined, requiring an inoculum of 6% to achieve a total removal of 90% in 24 hours (Tan et al., 2014). This association could be more relevant at high dye concentrations (> 100 mg/L) (Chen and Ting, 2015). However, in this study, no direct relationship between biomass growth and dye removal could be established, even with dye concentrations up to 500 ppm.
When evaluating the effect of the components of the culture medium (glucose 2-10 g/L; yeast extract 0-0.2 g/L; ammonium sulfate 0-1 g/L) on biomass production and dye removal, no effect was observed. These results do not agree with other studies reporting that the addition of yeast extract favored the decolorization of Pichia occidentalis G1 by 96%, related to the promotion of cell growth (Song et al., 2017). A study by Tan et al. (2014), showed that excess ammonium sulfate did not affect decolorization, although glucose concentrations above 4 g/L considerably decreased the removal capacity of the azo dye Acid Red B.
On the other hand, dye concentration and pH did have an effect on the percentage of removal by H. opuntiae, where an inversely proportional relationship was observed between these variables and the percentage of removal. Increasing the concentration of the dye Novasyn blue light BLR resulted in decreased yeast decolorization efficiency (80% at 250 ppm and 30% at 500 ppm), results that agree with previous studies where concentrations above 100 mg/L cause negative effects on decolorization (Tan et al., 2013; Guo et al., 2019) due to saturation of sorption sites in yeast as the dye concentration increases. Other studies support these results, Aksu (2003) reported that all yeast species evaluated were able to remove more than 90% of the dye at 100 ppm. However, above 400 ppm, this percentage decreased considerably because they had reached their saturation capacity. Cell wall composition can significantly influence dye binding capacity because functional groups capable of binding dye molecules, such as amino, carboxylic, sulfhydryl, phosphate, and thiol groups, differ in their affinity and specificity for dye binding (Volesky and May, 1995; Aksu and Dönmez, 2001; Fu and Wang, 2011). pH is another critical factor in dye removal, being favored at low pH conditions. The electrostatic attractive force of the biosorbent cell membrane and the dye is much higher under acidic conditions, resulting in much higher removal power. Mahmoud (2016), reported total decolorization when pH was between 1.0 and 2.0; furthermore, considerable decrease in dye removal as pH increased (70% removal at pH 3.0 and 20% at pH 6.0). These results are in agreement with the findings of this study, demonstrating a significant effect of pH on the capacity of dye removal by yeasts.
After determining the decrease in the decolorization of the Novasyn blue light BLR dye at high concentrations, the toxicity of this compound to H. opuntiae was evaluated. Concentrations up to 2000 ppm of dye were evaluated, evidencing a decrease in the removal capacity proportional to the increase in dye concentration (77.5 to 24.7%) (Figure 3). Although no direct relationship was found between biomass production and discoloration, higher concentrations of the dye also affected biomass production, although without completely inhibiting yeast growth. The presence of the dye is detected by H. opuntinae as a stress condition and a higher concentration may affect cellular processes unrelated to cell viability. Some studies suggest that an increase in dye concentration leads to an increase in the amount of time required for dye removal and thus decreases the potential for color removal (Surwase et al., 2013).
Once the removal capacity of H. opuntinae and its tolerance to high dye concentrations were proven, a decolorization test was carried out in wastewater from a textile effluent in which a decrease in the percentage of removal was evidenced (Figure 4A). Wastewater may contain salts, metals and other compounds to improve dye adhesion to fibers. However, these compounds are not biodegradable and can be toxic to the microorganism (Imran et al., 2014). Cations such as Na+, K+, Cu2+ and Ca2+ are the most common ions in textile wastewater and the presence of these ions leads to high ionic strength, which can significantly affect the performance of removal processes such as biosorption, weakening the electrostatic attraction between biomass and dye (Yu et al., 2009). The results of this assay show lower removal percentages than other studies, which could be related to different operational conditions, since Sinha et al. (2018) reported dye removal of 65% in wastewater with Candida sp. after 96 hours of exposure. Yeast immobilization provides a barrier between the effluent to be decolorized and the cells, protecting them from the toxic effects of the compounds present in the wastewater, reaching high removal percentages (95%) between 48 and 60 hours (Kurade, et al., 2019).
According to the UV-VIS spectra of the decolorized supernatant (Figure 4B), the absorbance peak of the dye Novasyn blue light BLR decreases considerably after 24 hours of contact with H. opuntiae, without observing the appearance of new peaks associated with the degradation of the azo ring. The decrease of the dye absorption peak without biomass pigmentation suggests that the mechanism of decolorization is by biodegradation (Song, et al., 2017). However, analysis of the supernatants with quantitative techniques such as HPLC-MS and FT-IR is necessary to determine the dye degradation.
The main enzymes involved in the biodegradation of azo dyes are azoreductases and ligninolytic enzymes such as laccases and peroxidases. Several studies have evaluated the enzymatic activity under different conditions, demonstrating that there is no single route of dye degradation and highlighting the importance of isolation and characterization of new fungal strains (Guo, et al., 2019).
In conclusion, contaminated niches such as wastewater are an important source of microorganisms with superior biotechnological characteristics that contribute to bioremediation processes, selecting populations with the ability to withstand and remove pollutants from the environment. In this study we were able to isolate a yeast capable of degrading the blue dye Novasyn blue light BLR in 24 hours, which was identified as H. opuntiae. Although this yeast has not been reported in the decolorization of compounds used in the textile industry, it demonstrated a great removal capacity and high tolerance to large concentrations of the dye in synthetic media. The results of this study demonstrate the complexity of the physiological processes associated with dye removal, which involve not only the concentration of the compound but also other factors such as temperature and pH of the medium, in addition to other factors present in the wastewater that could not be identified. Therefore, further studies are needed to identify the specific degradation mechanism and enzymes involved in this process in order to optimize its performance in textile effluents.

















