Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Caldasia
Print version ISSN 0366-5232
Caldasia vol.35 no.1 Bogotá Jan./June 2013
Producción de hojas y de inflorescencias de la palma de vino (Attalea butyracea) en el Valle seco del río Magdalena, Colombia
INGRID OLIVARES
GLORIA GALEANO
Faculteit der Aard- en Levenswetenschappen, Vrije Universiteit, 1081 HV Amsterdam, The Netherlands.i.l.olivaresaroca@student.vu.nl
Instituto de Ciencias Naturales, Universidad Nacional de Colombia, Apartado 7495, Bogotá D.C., Colombia.gagaleanog@unal.edu.co
ABSTRACT
The leaves of the wine palm (Attalea butyracea) are collected and harvested along the Magdalena River Valley in Colombia. Young leaves are used as a ceremonial symbol on Palm Sunday, and expanded leaves are used for thatching and for making handicrafts. In order to document leaf production and to evaluate the effect of leaf extraction on growth and development, we marked 80 individuals under extractive conditions and 40 individuals under non-extractive conditions and we followed leaf production during seven months. We also studied inflorescence production for one year, to evaluate the potential of A. butyracea as a source of sap for sugar manufacture. Leaf production in juveniles and sub-adults was correlated to the number of expanded leaves. Leaf production in adults was correlated to the number of expanded leaves and to stem size. Palms flower throughout the year, and several inflorescences develop simultaneously. The flowering peak occurs during the drier season. Inflorescence production was correlated to the stem height and to the number of expanded leaves, and it is probably affected by leaf harvest. We recommend leaf extraction only from individuals with stem over 3 m and with more than 25 expanded leaves. Inflorescence production of A. butyracea gives the palm a potential for sap extraction.
Key words: Arecaceae, Attalea butyracea, inflorescence production, leaf production, wine palm.
RESUMEN
En el valle seco del río Magdalena en Colombia se cosechan las hojas de la palma de vino (Attalea butyracea) para la comercialización. Los cogollos son usados como Ramo Bendito durante la Semana Santa, y las hojas expandidas para la construcción de techos y de artículos artesanales. Con el fin de documentar la producción de hojas y evaluar el efecto de su extracción sobre el crecimiento y el desarrollo, marcamos y monitoreamos durante siete meses 80 individuos en un área de reserva no cosechada y 40 individuos en un potrero donde se realiza cosecha. Adicionalmente, estudiamos la producción y el desarrollo de inflorescencias durante un año, para evaluar el potencial de A. butyracea para la producción de savia como fuente de azúcar a partir de las inflorescencias. La producción de hojas en individuos juveniles y subadultos estuvo correlacionada con el número de hojas expandidas, y en adultos estuvo correlacionada con el número de hojas expandidas y con la altura del tallo. Las palmas florecen a lo largo del año y producen varias inflorescencias simultáneamente. La producción de inflorescencias estuvo correlacionada con la altura y el número de hojas expandidas, y probablemente es afectada por la cosecha de hojas. Recomendamos cosechar únicamente las hojas de las palmas que tengan tallo mayor a 3 m y más de 25 hojas expandidas. La producción de inflorescencias sugiere que A. butyracea tiene potencial para la extracción de savia a través de las inflorescencias.
Palabras clave: Arecaceae, Attalea butyracea, palma de vino, producción de inflorescencias, producción de hojas.
Recibido: 30/01/2012
Aceptado: 19/04/2013
INTRODUCTION
The wine palm, Attalea butyracea (Mutis ex L. f.) Wess. Boer, is abundant and widespread in Colombia (Galeano & Bernal 2010), where it is used for a variety of purposes (Bernal et al. 2010). The leaves are used for thatch in rural communities and for ceremonial objects on Palm Sunday; at some localities, they are also used as raw material for the production of hats and other handicrafts (Bernal et al. 2010). Some of the uses of this palm are quite profitable and could help guaranteeing its long term conservation. Pulgarín & Bernal (2004) documented the production of wine from sap tapped from the trunk of A. butyracea. Through a cavity made at the meristematic area, a mean of one liter/day of sap is collected from every palm, for a maximum of 20 days. This use suggests A. butyracea's potential as a source of sugar, if the current destructive practice is replaced by the process of extracting the sap through the inflorescences as was discuss by Bernal et al. (2010). Because of its current and potential uses, A. butyracea might be an important resource for rural development in Colombia; however, knowledge on its biology is still limited.
In order to obtain biological information required to establish management plans for A. butyracea, we studied vegetative and reproductive phenology in two sub-populations of this species in the dry Magdalena River Valley, where it is still an important component of the ecosystems. An understanding of leaf production rate is necessary to determine which individuals are more suitable for leaf harvest with low impact. Information on inflorescence production rate, on the other hand, is important to estimate the potential of this palm as a sugar source.
MATERIALS AND METHODS
Attalea butyracea
This palm is known as palma de vino (wine palm), palma real or palma de cuesco. It is widespread in Colombia, along the Cauca and Magdalena river valleys, the Caribbean lowlands, the Eastern Plains and the Amazon region (Galeano & Bernal 2010). Its global distribution ranges from Mexico throughout Central America and into Colombia, Venezuela, Peru, Brazil, and Bolivia. Attalea butyracea is a solitary palm, with a stem 3–20 m tall and 25–80 cm diameter. The crown has 15–46 pinnate leaves, the petiole is short or absent and has numerous leaflets that are usually regularly arranged and spreading in the same plane. Inflorescences are borne among the leaves, and 2–5 are produced simultaneously with male and female flowers on the same plant, although inflorescences are predominantly unisexual; the peduncle is straight or curved 1–1.4 m long, surrounded by a woody, grooved bract 2–2.4 m long; the rachis is up to 1 m long, with 170–300 flowering branches 30–53 cm long; the male flowers have linear petals and six stamens. Fruits are oblong-ovoid or ellipsoid-oblong, 4.5–8.5 cm long and 3.0–4.5 cm diameter, yellow, with a woody endocarp and 1–3 seeds (Henderson et al. 1995, Galeano & Bernal 2010).
Study area
The study was carried out at Municipio de Nilo, Department of Cundinamarca (4°18' N, 74°36'W) in Colombia, in a landscape of low hills with a mean altitude of 450 m and inclinations ranging from 12 to 30%. Rainfall occurs in a bimodal regime, with an annual mean of 585 mm, peaking in March and September, and dry periods from June to August. The mean annual temperature is 27°C, and the mean annual relative humidity is 70%, with an increase in April, May and June. Mean annual solar radiation is about 2086 sunlight hours/year, which represents an average of six sunshine hours per day.
Despite continuous deforestation, the study area has an important population of wine palm. The local inhabitants harvest the leaves for thatching and they sell the young leaves for use on Palm Sunday. The use of young leaves for handicrafts, such as hats and fans, takes place only during local celebrations. According to the inhabitants, leaves from A. butyracea have been harvested for several decades in this area without a major effect on the population's persistence.
We selected two sample sites under different extractive conditions: harvest and non-havest of leaves in order to compare individual growth under both conditions. The first site is located in the Sumapaz River basin at 710 m elevation and it is locally known as finca Caimo-Volcanes (4°19'N, 74°37W). This area supports a secondary forest of 46 ha that has been protected from natural resources exploitation since 2005, and its structure is in recovery, as indicated by the presence of a dense vegetation of herbs and shrubs. A. butyracea dominates the site, forming a 15 m height canopy with a density of 70 adults/ ha, with abundant seedlings and juveniles.
The second sample site is a privately owned pasture, located also in the Sumapaz River at 4.7 km from the first site and 400 m elevation (4° 20 'N, 74°34' W). This area is used for cattle raising, and although palms were once abundant, they are now scattered under full sunshine with a density of 18 adults/ha. Once a year, palms from this pasture are harvested for Palm's Sunday. For this purpose young leaves from palms in all size classes are harvested; from each palm 1–2 young leaves are cut 25 cm above the bud. Once a year, only leaves from adult palms are harvested for thatching; for this use, all expanded leaves (15-46 leaves) are cut from each harvested palm, leaving only the young unexpanded leaves. The number of palms harvested for young leaves depends on the demand; in this pasture all palms present are harvested for that use. On the other hand, the number of palms harvested for thatching varies according to roof size and it ranges from 40-60 palms/roof.
Leaf production
In order to measure leaf production, we defined four size classes: seedlings (individuals with undivided leaves); juveniles (individuals without stem and up to 8 pinnate leaves); subadults (individuals with stem covered by old leaves from ground level to the crown and without evidence of reproduction); adults (individuals with several leaves, visible stem and evidence of reproduction). In the forest we marked 80 individuals in all size classes, 20 individuals per size class, whereas in the pasture we marked 20 subadults and 20 adults. We marked the youngest leaf of each individual and followed leaf production monthly from mid-March until mid-October 2009 (except for the size class "seedlings", which was followed only for five months, since a fire caused significant damage), registering the number of new leaves appearing during each time interval; additionally, we measured height, diameter at breast height and counted number of expanded leaves for each individual.
Inflorescence production
Flowering and fruiting were followed in 23 individuals in the forest and 31 individuals in the pasture. From March 2009 until February 2010, every month we recorded for each individual the number of buds and inflorescences in each of the following phases: anthesis, unripe and ripe fruits. Palms usually have more than one inflorescence simultaneously, at different phases. For that reason, in the analysis we referred to the number of reproductive structures, which corresponds to the total number of buds, inflorescences and infructescences present in each sample during each time interval. Since the complete cycle from buds to ripe fruits can take more than one year, we could not – in the time available – follow the whole process in our samples; hence, we calculated the range and the average time of each phase (bud, anthesis, unripe and ripe fruits) and then we extrapolated these to obtain an average time for the whole process.
Data Analysis
In order to describe the general characteristics of each size class, we calculated the average stem height and diameter (in adults), the number of non-expanded leaves and the number of expanded leaves, and we tested for differences between samples. We determined leaf production rate/individual/month during seven months. Studies have shown that leaf production in palms changes according to the life stage (seedlings, juveniles subadults and adults), but for each of them leaf production is constant throughout the year (Piñero et al. 1984; Lugo & Rivera 1987). Thus, we extrapolated the seven months rate to an annual production value. We compared annual leaf production for both sites and all size classes by a multiple rank test. We also determined annual inflorescence production/individual/month for each site and then we tested for differences by a Student's t-test.
To evaluate the relationship between variables, we carried out a correlation analysis, which shows which variables have more influence on leaf production and inflorescence production. The data analyses were performed using the software STATGRAPHICS Centurion XV. Version 15.2.06. (©Statpoint 1982-2009).
To estimate the palms'age, we used the relationship between leaf production and the number of leaf scars, as it has been done in previous studies with palms (Bernal 1998; Rodríguez et al. 2005); we calculated the age of palms at different life stages: seedlings, juveniles and adults. For germination we used data obtained by Harms & Dalling (1995); they established an average time of 10 months. For seedlings and juveniles we used the relation between age and the increase in the number of veins with each new leaf produced; whereas in adults we used the number of leaf scars on the stem.
RESULTS
General characteristics and leaf production
Stems were thicker (t = 6.76413, p=5.13692E-8) and the number of expanded leaves/individual in adults and subadults (F=36.02; p<0, 00001) was significantly higher in palms of the pasture (Figure 1). Although the differences were not significant, on average stems were higher and there were more unexpanded leaves in palms in the pasture (Tabla 1). Annual leaf production was constant throughout the seven months but differed in all size classes (Figure 2; F=69.67, p<0.00001), and it was highest in the pasture in adults and particularly in subdadults, which produced as many leaves per year as adults in the forest and significantly more leaves than subadults in the forest.


The number of expanded leaves is not related to stem height, but all the variables are positively related to annual leaf production in adults (Tabla 2). On average, palms less than 3 m high and with less than 25 leaves had the lowest annual leaf production (< 4 leaves/year) (Figure 3). In juveniles and subadults, annual leaf production was also related to the number of expanded leaves (r=0, 6301, p<0, 00001, n=60).

Reproductive phenology
Inflorescence production in the forest was not significantly different from the pasture. In the forest, average annual inflorescence production per individual was 6.4 (range 1.7–12), positively related to the number of expanded leaves (r=0.7802, p=0.0004) and weakly related to stem height (r=0.5709, p=0.0209) (Figure 4). In the pasture, average annual inflorescence production was 7.3 (range 2.0–22) and not correlated to number of leaves or stem height.

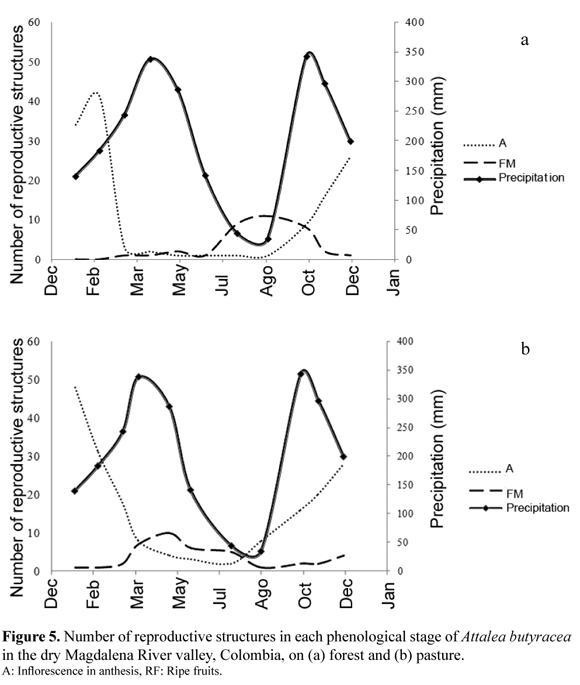
During the sampling period the percentage of individuals with fruits was 70% in the forest and 66% in the pasture, but development of reproductive structures was different at the two habitats (Figure 5). In both habitats the flowering peak (A) occurred in December–January, but there were most ripe fruits in the forest in the dry season (July–August) and more ripe fruits in the pasture in the beginning of the wet season (March–May). In the pasture the average time between the first evidence of floral buds (approx. 30 cm long) and fruit fall was 6.8 months, whereas in the forest this process took in average 9.0 months.
Growth
Age estimation showed that the seedling stage lasts 5-13 years, the juvenile stage lasts 15–27 years, which is followed by 14–18 years that it takes to produce a 5 m tall stem; thus, a palm with a 5 m tall stem, has an estimated age of 35–59 years (this value includes the 10 months germination period estimated by Harms and Dalling (1995).
DISCUSSION
General characteristics
Although stem height and diameter and the number of young leaves were not significantly different between palms growing in forest and in pasture, adult and subadult palms in the pasture were, on average, higher, probably because leaf production in palms involves an increase in height (Henderson 2002); thus we expected palms on the pasture to be taller because of their higher leaf production. On the other hand, several studies have shown that palms, in general, do not have evident increase in diameter after establishment growth (Dransfield et al. 2008, Henderson 2002). Nevertheless, we found a positive correlation between annual leaf production and stem height and diameter. Since the pasture is located at a lower elevation, and palms at this site have higher availability of sunlight, it is possible that microclimatic conditions have an influence on stem size-leaf production correlation. The influence of microclimatic conditions on individual growth has been shown in several studies with different palm species including A. butyracea (Rich et al. 1995, Svenning 2001).
Juveniles and subadults of A. butyracea have high sunlight requirements; hence, the number of expanded leaves in these palms depends on their exposure to sunlight, as found for this species at Barro Colorado Island (Araus & Hogan 1994). Taller stems provide access to better light conditions, which increases leaf production, as documented for A. butyracea (as Scheelea zonensis L.H.Bailey; Hogan 1986) and Astrocaryum mexicanum Liebm. ex Mart. (Mendoza et al. 1987). This explains the relation between crown size and stem height.
Leaf production
Leaf production in seedlings, juveniles and subadults at each site depended only on the number of expanded leaves. Variance within each size class was high, probably due to the strong influence of light conditions on individual growth, as has been recorded for the same species in Panama (Hogan 1986) and for other species such as Euterpe edulis Mart. (Carvalho et al. 1999). Leaf production of A. butyracea is directly proportional to the stem height and the number of expanded leaves, which may be related to light availability (De Steven et al. 1987); however, as our findings showed, under favorable light conditions stem height is not a limiting factor, as subadults in the pasture reached the same leaf production as adults in the forest.
Studies on the different palm genera, suggest that Attalea is one of the palm genera with the highest leaf production (De Steven et al. 1987, Echeverry 1993, Henderson 2002). The range of leaf production in our study (3–12 leaves/year), is comparable to that found in other large species of Attalea, such as A. colenda, on the Ecuadorean coast (9.1 leaves/year; Feil 1996), and A. phalerata, at the rain forest in Bolivia, (6–8 leaves/year; Paniagua 1998); and it contrasts to the lower leaf production in acaulescent species as A. amygdalina, at a secondary forest in Colombia (2 leaves/year; Ruíz-Echeverry 1984).
Reproductive phenology
Several studies in palms have shown that inflorescence production is directly proportional to light, water and soil nutrients availability (Martínez-Ramos et al 1988; Velarde & Moraes 2008). However, in the pasture, where light conditions are more favorable, the production of inflorescences was not significantly different from that in the forest, and it was not correlated to other variables (stem size and number of expanded leaves). Therefore we suggest that leaf harvest might decrease reproduction of palms in the pasture, because in order to compensate the loss of tissue, plants would allocate a large part of their resources to produce new leaves, as has been shown in several studies of other palm species (Rockwood 1973, Oyama & Mendoza 1990, Ratsirarson et al.1996).
The wine palm produces flowers and fruits throughout the year; however, we observed flowering and fruiting peaks. On both pasture and forest the flowering peak was evident at the beginning of the year, during a period of low precipitation. In the Eastern Plains of Colombia A. butyracea also flowers throughout the year but the peak of flowering was weak and occurred between August and October, during the rainy season (Mesa & Romero 2008); the same pattern was found in Panama, where flowering of A. butyracea take place mainly in the latter part of the rainy season (De Steven et al. 1987). These differences could be explained by the differences between rainfall patterns in these regions (bimodal in the Magdalena Valley and unimodal in the Eastern Plains as well as in BCI in Panama). On the other hand, we also found ripe fruits throughout the year, with weak peaks for each habitat at different times of the year. In the forest, most ripe infructescences occurred in August, at the beginning of the second rainy season, whereas in the pasture they occurred between March and April, during the first rainy season. The reproductive cycle was shorter in the pasture. Adler and Lambert (2008) concluded that climatic variability at local scale has a strong influence on fruiting patterns of Attalea butyracea; therefore, it is possible that light and altitude conditions stimulate the reproductive cycle in the pasture, as it has been found for Astrocarym mexicanum (Martínez-Ramos et al.1988).
Effect of harvest
Experiments have shown that defoliation in palms produces a redistribution of resources towards new leaf production, which compromises the growth and development of other structures, causing an effect on growth and reproduction, which depends in part, on the level of harvest. (Mendoza et al. 1987, Oyama & Mendoza 1990, Chazdon 1991, O'Brien & Kinnaird 1996, Ratsirarson et al. 1996, Anten & Ackerly 2001, McKean 2003, Endress et al. 2004). While the results presented here do not test directly the effect of leaf harvest on palms morphology, for which harvesting essays as well as sampling under similar environmental conditions are a requisite, our findings indicate that this activity could influence stem height, number of leaves and leaf production of A. butyracea. Sampling under similar environmental conditions is crucial, because environmental conditions could be more important to explain variability in vegetative characteristics of palms, but also because favorable conditions of light in the pasture could reduce the effect of harvest on palm's growth and hence, act as a confounding factor. On the other side, the effect of harvest on reproduction is probably explained by unmeasured variables, like the proportion of male to androgynous inflorescences, the proportion of inflorescences that developed into infructescences, fruit size and seed production (Rockwood 1973, Oyama & Mendoza 1990, Kinnaird 1992).
Attalea butyracea has been suggested as a promising source of oil, sugar, and biofuel (Bernal et al. 2010). Current sap tapping threatens palm populations, because it implies the palm's death; however, sap tapping through the inflorescences is well known for several palm species in Asia (Arenga pinnata, Borassus flabellifer, Caryota urens, Cocos nucifera, Phoenix sylvestris), and this sap is used for the production of sugar, a product commercialized in local and international markets (Dransfield 1976, De Zoysa 1992, Everett 1995). Therefore, the evaluation of alternative methods of sap tapping for A. butyracea is prioritary. If sap extraction through the inflorescences was achieved for this species, then the presence of inflorescences throughout the year would be a good starting point.
Regarding current management of A. butyracea in Colombia, which is focused mainly on leaf harvest, we do not recommend harvesting young palms, because their leaf production is low, and therefore harvest can cause a negative effect on growth and future reproduction. Based on our findings, we recommend harvesting adults with stem height over 3 m and more than 25 expanded leaves, which have the highest leaf production rate (5–12 leaves/year); this would decrease the effect of harvest.
Additionally, we recommend the introduction of A. butyracea in agrosilvopastoral systems, as a sustainable way of management. Planted at low density (at least 18 adults/ha, as it is in the sampled pasture), where sunlight conditions are favorable for pastures and other fodders, leaf harvest and inflorescences tapping could be possible with a low effect on the survival and reproduction. It is also important to protect natural forests, where individuals in all size classes develop and to guarantee the constant production of fruits, which are vital for the persistence of entire ecosystems, because palms fruits and seeds are fundamental gears for several soil-plant-animal interactions.
ACKNOWLEDGMENTS
We thank Corporación Autónoma Regional de Cundinamarca (CAR), Brigada Forestal de Agua de Dios (Cundinamarca), Alcaldía de Nilo (Cundinamarca), and PALMS Project FP-7 No. 212631, for their support on the fieldwork. To Ávila Garzón's family for allowing us to work on his farm and all the facilities they provided us. We also thank Rodrigo Bernal for his valuable suggestions on the manuscript, his intellectual support and his guidance on the fieldwork, Henrik Balslev for his valuable suggestions on the manuscript, and two anonymous reviewers for their critical evaluation of the manuscript.
LITERATURE CITED
1. Adler, G.H. & T.D Lambert. 2008. Spatial and temporal variation in the fruiting phenology of palms in isolated stands. Plant Species Biology 23: 9-17. [ Links ]
2. Anten, N.P.R. & D.D. Ackerly. 2001. Canopy-level photosynthetic compensation after defoliation in a tropical understorey palm. British Ecological Society 15: 252-262. [ Links ]
3. Araus, j. L. & k. P. Hogan.1994. Leaf structure and patterns of photoinhibition in two neotropical palms in clearings and forest understory during the dry season. American Journal of Botany 81: 726-738. [ Links ]
4. Bernal, R. 1998. Demography of the vegetable ivory palm Phytelephas seemanii in Colombia, and the impact of seed harvesting. Journal of Applied Ecology 35: 64-74. [ Links ]
5. Bernal, R., G. Galeano, N. García, I.L. Olivares Y C. Cocomá. 2010. Uses and Commercial prospects for the wine palm, Attalea butyracea, in Colombia. Ethnobotany Research and Applications 8: 255-268. [ Links ]
6. Carvalho, R.M., F.R. Martins & F.A.M. Santos. 1999. Leaf ecology of pre-reproductive ontogenetic stages of the palm tree Euterpe edulis Mart. (Arecaceae). Annals of Botany 83: 225–233. [ Links ]
7. Chazdon, R. 1991. Effects of leaf and ramet removal on growth and reproduction of Geonoma congesta, a clonal understory palm. Journal of Ecology 79: 1137-1146. [ Links ]
8. De Steven, D. D.M. Windsor, F.E. Putz & B. De Leon. 1987. Vegetative and reproductive phenologies of a palm assemblage in Panama. Biotropica 19: 342-356. [ Links ]
9. De Zoysa, N. 1992. Tapping patterns of the kitul palm (Caryota urens) in the Sinharaja area, Sri Lanka. Principes 36:28-33. [ Links ]
10. Dransfield, J. 1976. Palm sugar in East Madura. Principes 20: 83-90. [ Links ]
11. Dransfield, J., N.W. Uhl, C.B. Asmussen, W.J. Baker, M.M. Harley, C.E. Lewis. 2008. Genera Palmarum. Royal Botanic Gardens, Kew. [ Links ]
12. Echeverry, A. 1993. Arquitectura y desarrollo de diez especies de palmas y Phenakospermum guianense en sucesión secundaria: formación Araracuara, Caquetá, Colombia. Departamento de Biología. Facultad de Ciencias Exactas y Naturales. Universidad de Antioquia. Medellín. Trabajo de grado. [ Links ]
13. Endress, B.A., D.L. Gorchov, M. B. Peterson & E.P. Serrano. 2004. Harvest of the palm Chamaedorea radicalis, its effects on leaf production, and implications for sustainable management. Conservation Biology 18: 822-830. [ Links ]
14. Everett, Y. 1995. The kitul palm: ethnobotany of Caryota urens L. in highland Sri Lanka. Journal of Ethnobiology 15: 161-176. [ Links ]
15. Feil, J.P. 1996. Fruit production of Attalea colenda (Arecaceae) in Coastal Ecuador- an alternative oil resource? Economic Botany 50: 300-309. [ Links ]
16. Galeano, G. & R. Bernal. 2010. Palmas de Colombia. Guía de Campo. Editorial Universidad Nacional de Colombia. Instituto de Ciencias Naturales, Facultad de Ciencias-Universidad Nacional de Colombia, Bogotá D.C. [ Links ]
17. Harms, K.E. & J.W. Dalling. 1995. Observations on the seasonal consistency in germination timing for Scheelea zonensis. Principes 39: 104-106. [ Links ]
18. Henderson, A., G. Galeano & R. Bernal. 1995. Field Guide to the Palms of the Americas. Princeton University Press. New Jersey. [ Links ]
19. Henderson, A. 2002. Evolution and Ecology of Palms. The New York Botanical Garden Press. New York. [ Links ]
20. Hogan, K.P. 1986. Plant architecture and population ecology in the palms Socratea durissima and Scheelea zonensis on Barro Colorado Island, Panama. Principes 30: 105-107. [ Links ]
21. Kinnaird, M. 1992. Phenology of flowering and fruiting of an East African riverine forest ecosystem. Biotropica 24: 187-194. [ Links ]
22. Lugo, A.E. & T. Rivera. 1987. Leaf Growth Rate, and Age of the Palm Prestoea Montana in the Experimental Forest, Puerto Rico. Journal of Tropical Ecology, 3:151-161. [ Links ]
23. Mckean, S. 2003. Toward sustainable use of palm leaves by a rural community in Kwazulu-Natal, South Africa. Economic Botany 57: 65-72. [ Links ]
24. Martínez-Ramos, M., J. Sarukhan & D. Piñero. 1988. The demography of tropical trees in the context of forest gap dynamics: the case of Astrocaryum mexicanum at Los Tuxtlas tropical rain forest. Págs. 293-313 en: Davy A. J., M. J. Hutchings & A. R. Watkinson (eds.). Plant Population Ecology: The 28th Symposium of the British Ecological Society, Sussex. Blackwell Scientific, Oxford. [ Links ]
25. Mendoza, A., D. Piñero & J. Sarukhan. 1987. Effects of experimental defoliation on growth, reproduction and survival of Astrocaryum mexicanum. Journal of Ecology 75: 545–554. [ Links ]
26. Mesa, M.S. & L.E. Romero. 2008. Comparación de la biología reproductiva y ecología de la polinización de Attalea butyracea y Attalea insignis (Palmae) en Casanare Colombia. Programa de Biología. Fundación Universitaria Internacional del Trópico Americano UNITROPICO. Yopal. Tesis. [ Links ]
27. O'brien, T. G. & M. F. Kinnaird. 1996. Effect of harvest on leaf development of the Asian palm Livistona rotundifolia. Conservation Biology 10: 53-58. [ Links ]
28. Oyama, K. & A. Mendoza.1990. Effects of defoliation on growth, reproduction, and survival of a neotropical dioecious palm, Chamaedorea tepejilote. Biotropica 22: 119–123. [ Links ]
29. Paniagua, N.Y. 1998. Estudio comparativo de la densidad y los niveles de producción de hojas, frutos y semillas en poblaciones naturales de Attalea phalerata (Palmae) sometidas a diferente intensidad de extracción (Riberalta, Depto. Beni, NE Bolivia). Carrera de Biología. Facultad de Ciencias Puras y Naturales. Universidad Mayor de San Andrés. La Paz. Tesis. [ Links ]
30. Piñero, D., M. Martínez-Ramos & J. Sarukhan. 1984. A population model of Astrocaryum mexicanum and a sensitivity analysis of its finite rate of increase. Journal of Ecology 72: 977-991. [ Links ]
31. Pulgarín, N. & R. Bernal. 2004. El potencial de la palma de vino, Attalea butyracea, como planta azucarera. Pág.194 en: Ramírez-Padilla, B.R., Macías-P. D., Varona-B. G. (eds.). Libro de Resúmenes Tercer Congreso Colombiano de Botánica. Universidad del Cauca, Popayán. [ Links ]
32. Ratsirarson, J., J. A. Silander & A. F. Richard. 1996. Conservation and management of a threatened Madagascar palm species, Neodypsts decaryi, Jumelle. Conservation Biology 10: 40-52. [ Links ]
33. Rich, P.M., N. M. Holbrook & N. Luttinger. 1995. Leaf development and crown geometry of two Iriarteoid palms. American Journal of Botany 82: 328-336 [ Links ]
34. Rockwood, L.L. 1973. The effect of defoliation on seed production of six Costa Rican tree species. Ecology 54: 1363-1369. [ Links ]
35. Rodríguez, B. S., M.A. Orjuela & G. Galeano. 2005. Demography and life history of Geonoma orbignyana: An understory palm used as foliage in Colombia. Forest Ecology and Management 211: 329-340. [ Links ]
36. Ruíz-Echeverry, L. 1984. Contribucion al conocimiento de la palma de almendron Attalea victoriana Dugand en su medio natural. Cespedesia 13: 49-50. [ Links ]
37. Svenning, J.C. 2001. On the role of microenvironmental heterogeneity in the ecology and diversification of neotropical rain-forest palms (Arecaceae). Botanical Review 67: 1-53. [ Links ]
38. Velarde, M.J. & M. Moraes. 2008. Densidad de individuos adultos y producción de frutos del asaí (Euterpe precatoria, Arecaceae) en Riberalta, Bolivia. Ecología en Bolivia 43: 99-110. [ Links ]















