Introduction
At the end of 2019, new pneumonia emerged in Wuhan, China, due to the SARS-CoV-2 virus (Severe Acute Respiratory Syndrome-Coronavirus-type 2) whose spread has not been interrupted so far, representing a health crisis that reached pandemic epidemiological status in March 2020 1. According to the World Health Organization (WHO), more than 350 million cases and about 5.5 million deaths have been reported worldwide as of the date of conception of this manuscript 2. The global crisis has prompted the development of multiple vaccines to reduce the impact of the virus.
Clinical trials of the Pfizer/BioNTech vaccine have demonstrated a favorable safety profile and efficacy for the prevention of COVID-19 3. Local and systemic reactions following vaccine administration have been reported, mainly fever, headache, fatigue, chills, myalgias and arthralgias 4,5; however, myocarditis, immune thrombotic thrombocytopenia, Guillain-Barré and autoimmune hepatitis have been reported infrequently 5,6. Hepatocellular injury has also been described in association with SARS-CoV-2 vaccination in the absence of autoimmunity 7. Manifestations of liver injury range from serum elevation of alanine transferase (ALT), aspartate transferase (AST) and bilirubin enzymes to acute liver failure in severe cases 8.
The following is the case of a 22-year-old female patient who developed drug-induced liver injury (DILI) following the first and second doses of Pfizer/BioNTech vaccine. The purpose of this article is to encourage clinical suspicion for adequate surveillance and monitoring of patients who may manifest hepatic involvement and if necessary, it is recommended not to apply this type of vaccine and to replace it with those with a mechanism of action other than RNAm.
Case description
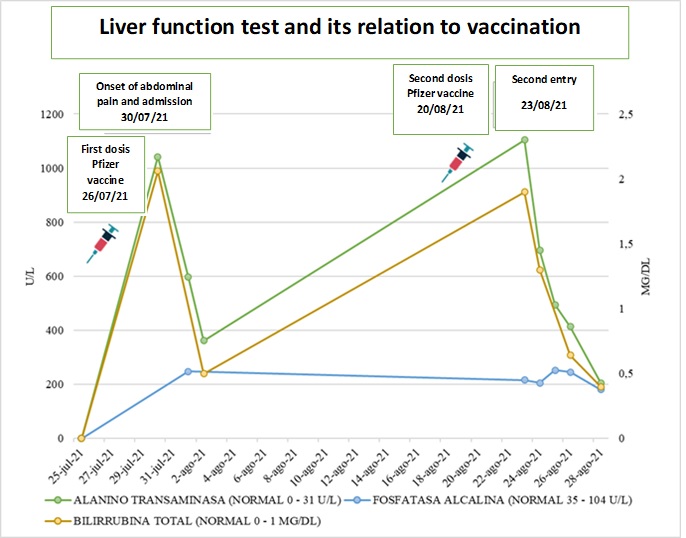
Female patient, 22 years old, of mixed race, from the urban area of the city of Cali, who works in a stationery store. Arrived at the emergency department for six days with colicky abdominal pain in the right hypochondrium and epigastrium, associated with emesis of food content, with no changes in urine or stool. In her medical history, she reported received the first dose of Pfizer-BioNTech vaccine (RNAm) for COVID-19, six days before her admission. During the period before and after the vaccination she did not receive any analgesic such as acetaminophen or other analgesic medication after the two doses. She denies taking other hepatotoxic drugs, herbal or over-the-counter supplements, alcohol consumption and psychoactive substances. The patient reports no symptoms of COVID-19, epidemiological link with a positive patient confirmed by RT-PCR and negative test for SARS-CoV-2 virus identification. On physical examination, she was stable, afebrile, with anicteric sclerae and mucous membranes. She presented abdominal pain on deep palpation in the epigastrium, without signs of peritoneal irritation, stool and urine without changes in color and composition. Elevated transaminases (TSA 1,050 U/L (0-32), TLA 1,042 U/L (0-31), elevated bilirubins TB 2.06 mg/dL (0-1) DB 1.94 mg/dL (0-0.3), and alkaline phosphatase (AP) 248 U/L (35-104), twice its normal value, with amylase and studies for hepatotropic viruses, anti-nuclear antibodies (ANA) and anti-smooth muscle negative (Figure 1). Biliary tract ultrasound and cholangioresonance were normal. Two days after admission the patient presented clinical improvement, with normalization of liver tests and was discharged.
The patient consulted for similar clinical symptoms 21 days after the first dose and after the second dose of Pfizer vaccine 3 days ago. During this period, she did not ingest any analgesic, (denied using including acetaminophen and NSAIDs or hepatotoxic and herbal substances). Laboratory tests showed hepatocellular injury (AST 1,128 U/L (0-32), ALT 1,104 U/L (0-31), BT 1.9 mg/dL (0-1) BD 1.83 mg/dL (0-0.3) alkaline phosphatase 216 U/L (35-104), GGT 218 U/L (5-36) (Figure 1), with negative amylase and hepatotropic virus test. Within the autoimmunity test, she presented anti-smooth muscle antibodies in 1:80 but with negative antinuclear antibodies, anti-microsomal antibodies and serum immunoglobulin levels. Molecular testing for SARS-CoV-2 virus identification was not performed given the absence of symptoms. Liver and biliary tract ultrasound and cholangioresonance were unaltered. Treatment was started with simple hyoscine 20 mg orally every 8 hours and intravenous fluids as supportive therapy and expectant management. She presented improvement of abdominal pain and progressive decrease of transaminases and bilirubin levels 24 hours after admission, with normalization of these levels on the fifth day of hospitalization, for which she was discharged.
During the follow-up, she was evaluated four weeks later by gastroenterology with new laboratories including liver function tests and control anti-smooth muscle antibodies, which are within expected parameters and a probable diagnosis of DILI secondary to vaccination is confirmed.
The patient reports feeling satisfied with the medical staff for protecting her life and the care provided during her hospital stay.
Discussion
We present the case of a young patient without comorbidities who developed clinical and laboratory signs compatible with drug-induced liver damage with a hepatocellular pattern 9. To reach the etiological diagnosis, causes of moderate elevation of liver tests associated with the described pattern were ruled out in the absence of acute liver failure criteria, which included among the differential diagnoses: viral hepatitis, fatty liver associated with metabolic dysfunction, autoimmune hepatitis and hepatic alterations secondary to structural and obstructive processes, however, there was a diagnostic possibility of hepatotoxicity. Therefore, a complete interrogation was carried out, ruling out any history related to the consumption of drugs, including herbal supplements, analgesics such as acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs), as well as the consumption of alcohol or drugs of abuse, in order to search for the causal agent that could explain the hepatic damage. Given the temporal relationship with the previous vaccination with Pfizer-BioNTech vaccine (RNAm) for SARS-CoV-2, it was the triggering agent of the liver injury.
To establish causality between the secondary ADR and the vaccine, the algorithm of Naranjo et al 10 was used (Figure 1). In this sense, the efficacy and safety studies of the BNT162b2 Pfizer-BioNTech (RNAm) vaccine 11, which include the Latin population and evaluate the presence of adverse reactions with the two applications of the vaccine, show that local reactions were the most frequent, these only occurred in less than 1% of the cases. Among the adverse events reported, there were no reports of alteration of liver function tests or hepatotoxicity reactions. Likewise, when describing the population with liver disease, only 0.6% of the total patients were included 11. The safety and effectiveness studies of the other vaccines available for SARS-CoV-2 do not find adverse events related to hepatic pathology or involvement of the biliary tract 11-13; except for the study of the Moderna vaccine which reports <0.1% of cases of acute cholecystitis without hepatitis 14. In the specialized literature, some studies of hepatocellular lesions have been related to vaccination 15,16. In this order of ideas, in a study carried out in the United Kingdom, some alteration of hepatic tests was evidenced after vaccination with Pfizer/BioNTech BNT162b2 RNAm 15. Likewise, a description has been made of patients (13/16 cases) who received first or second doses of Pfizer or Moderna vaccine, who presented elevated transaminases with a hepatocellular pattern, three of whom developed acute liver injury 16. The authors of this study, supported by the proposal of other researchers and given the evidence in the literature, suggest the possibility of immune-mediated reactions against the Spike protein favoring an aberrant hepatic condition 16. On the other hand, Alqarni et al. and Mann et al. 17,18, report cases of drug-induced liver injury (DILI) following the Pfizer/BioNTech BNT162b2 mRNA vaccine very similar to the one described in this article.
According to the algorithm of Naranjo et al. 10, it was established that the adverse reaction occurred after the administration of the suspected drug, seven days after vaccination and reappeared three days after administering the second dose of the same vaccine, without other factors or underlying associated causes, with a similar clinical and laboratory picture (Figure 2).
Among the limitations of the study, it was not possible to establish the adverse event through objective tests such as a histopathological study by hepatic biopsy that would allow demonstrating changes of acute hepatic lesion related to hepatotoxicity 19. Similarly, there are no experimental studies that demonstrate this type of change in tissue by vaccines, only reports of cases of patients vaccinated for SARS-CoV-2, in which different inflammatory patterns were found with portal involvement or interface hepatitis attributed to the association of other drugs causing DILI in the patients studied 16. For this reason, based on the algorithm performed, it can be considered that the adverse reaction to the vaccine is probable (Figure 2).
Based on the above, we believe that the probable diagnosis of the patient is an adverse reaction drug (ARD) associated with vaccination, establishing the diagnosis of DILI given the hepatic involvement. Furthermore, a pattern of hepatocellular injury could be established based on the hepatic biochemistry test. In addition, there was an evident elevation of ALT 2 times above the normal limit and an R index greater than 5, R being the ratio between ALT and AP 20. Subsequently, the CIOMS/RUCAM (Roussel Uclaf Causality Assessment Method) scale (Table 1) is performed to determine the causality relationship, obtaining a score of 10 for a high probability of DILI 21. Finally, it is considered that due to the characteristics, the time of elevation of liver enzymes and the clinical manifestations of the patient after vaccination, it is an idiosyncratic type of DILI given its low frequency, the absence of a relationship with a defined dose, unpredictable and not reproducible in animal models so far 22.
Table 1 CIOMS/RUCAM Scale (Roussel Uclaf Causality Assessment Method)
| CIOMS/RUCAM | ||||||
|---|---|---|---|---|---|---|
| Type of liver injury | Hepatocellular | Value | Cholestatic/mixed | Value | ||
| Chronological criteria | First exhibition | Second exhibition | First exhibition | Second exhibition | ||
| Time from drug intake to symptom onset | 5-90 days | 1-15 days | 2 | 5-90 days | 1-90 days | 2 |
| <5 o >90 days | >15 days | 1 | <5 o >90 days | > 90 days | 1 | |
| Time of drug withdrawal at symptom onset | <15 days | >=15 days | 1 | <= 30 days | <= 30 days | 1 |
| Course of the disease | Difference between ALT maximum value and upper normal limit | value | Difference between maximum ALP value and upper normal limit | Value | ||
| Upon withdrawal of the drug | Improvement >50% in 8 days | 3 | Improvement >50% in 180 days | 2 | ||
| Improvement >50% in 30 days | 2 | Improvement <50% en 180 days | 1 | |||
| Lack of information or no improvement | 0 | Lack of information or no improvement | 0 | |||
| Worsening or <50% improvement in 30 days. | -1 | |||||
| Risk Factors | Age (≥55 years) | 1 | Age (≥55 years) | 1 | ||
| Alcohol consume | 1 | Alcohol consume or pregnancy | 1 | |||
| Concomitant therapy | None or unknown | 0 | None or unknown | 0 | ||
| Drug with suggestive contribution | -1 | Drug with suggestive contribution | -1 | |||
| Known hepatotoxicity, suggestive contribution | -2 | Known hepatotoxicity, suggestive contribution | -2 | |||
| Proven role in the case | -3 | Proven role in the case | -3 | |||
| No information available | 0 | No information available | 0 | |||
| Exclusion of other non-drug causes | Discarded | 2 | Discarded | 2 | ||
| Possible to not investigated | -2 a 1 | Possible to not investigated | -2 a 1 | |||
| Other probable cause | -3 | Other probable cause | -3 | |||
| Previous hepatotoxicity information | Unknown reaction | 0 | Unknown reaction | 0 | ||
| Published but not labeled on the drug | 1 | Published but not labeled on the drug | 1 | |||
| Labeled in the characteristics of the drug | 2 | Labeled in the characteristics of the drug | 2 | |||
| Response to Re-administration of the drug | Positive | 3 | Positive | 3 | ||
| Compatible | 1 | Compatible | 1 | |||
| Negative | -2 | Negative | -2 | |||
| Not available or not interpretable | 0 | Not available or not interpretable | 0 | |||
| Plasma concentrations known to be toxic | 3 | Plasma concentrations known to be toxic | 3 | |||
| Validated laboratory tests with good predictive values | Positive | 3 | Positive | 3 | ||
| Negative | -3 | Negative | -3 | |||
| Not available or not interpretable | 0 | Not available or not interpretable | 0 | |||
Source: Taken and adapted from Danan, G., & Teschke, R 22.
Similarly, it would have been proposed to perform liver biopsy in the hospital to evaluate changes related to hepatotoxicity and to rule out other etiologies such as autoimmune diseases in a young woman without comorbidities, as in the case presented. According to Kleiner et al. 19, liver biopsy is not required to evaluate patients with suspected DILI. However, it raises some guidelines for the performance of the biopsy and that it can be performed when it is considered whether the histopathological findings are due to DILI or it is believed in another pathology, if the biopsy contributes to clarifying which is the causal agent of DILI, if the administration of steroids resolves the severity of the injury and the inflammatory pattern and the inflammatory pattern is resolved by the administration of steroids and if it provides additional information regarding the patient's prognosis. Likewise, it suggests that DILI is a diagnosis of exclusion, emphasizing the identification of the causative agent and the correlation with the clinical history and laboratory tests. Similarly, he proposes that the histopathological changes are very varied, and their spectrum includes inflammation, necrosis, cholestasis, fibrosis, nodular regeneration, vascular injury and destruction of the biliary ductus, among others 19.
Regarding treatment, in case the patient's symptoms persist despite the fact that supportive measures such as hydration in conjunction with antispasmodics and liver tests remain elevated, a short course of low-dose steroids is suggested, which is described in the treatment of idiosyncratic DILI that does not spontaneously improve with withdrawal of the causative agent 22.
For this reason, it is suggested to evaluate the risk/benefit ratio according to the clinical context in case new applications of vaccines are required. For this reason, decisions should be individualized and the application of vaccines with a mechanism of action other than mRNA should be considered as an alternative.











 text in
text in