Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biosalud
Print version ISSN 1657-9550
Biosalud vol.15 no.1 Manizales Jan./June 2016
https://doi.org/10.17151/biosa.2016.15.1.4
DOI: 10.17151/biosa.2016.15.1.4
9-[(E)-2-phenylethenyl]anthracene AND 9-[(E)-2-(naphthalen-2-yl)ethenyl] anthracene AS TRAPS FOR SINGLET OXYGEN: PHOTOSENSITIZED OXIDATION AND PHOTODYNAMIC EFFECT ON Leishmania tarentolae PARASITES
Carlos Alberto Marín-Londoño1
Steve B. Brand2
Luz Amalia Ríos-Vásquez3
Rogelio Ocampo-Cardona4
Marjorie A. Jones5
David L. Cedeño6
1 MSc in Chemistry. Department of Chemistry, Universidad de Caldas. Manizales, Colombia. E-mail: carlosalbertomarin@yahoo.com ORCID: 0000-0002-6548-1306
2 MSc in Chemistry. Department of Chemistry, Illinois State University. Normal, Illinois, USA. E-mail: sbbrand2@gmail.com ORCID: 0000-0003-3526-851X
3 PhD in Chemistry Sciences. Department of Chemistry, Universidad de Caldas. Manizales, Colombia. E-mail: amalia.rios@ucaldas.edu.co ORCID: 0000-0002-4510-7771
4 PhD in Chemistry Sciences. Department of Chemistry, Universidad de Caldas. Manizales, Colombia. Corresponding author. E-mail: rogelio.ocampo@ucaldas.edu.co ORCID: 0000-0002-3375-6352
5 PhD in Chemistry Sciences. Department of Chemistry, Illinois State University. Normal, Illinois, USA. E-mail: majone3@ilstu.edu ORCID: 0000-0002-6306-0700
6 PhD in Chemistry Sciences. Department of Chemistry, Illinois State University. Normal, Illinois, USA. E-mail: daleceme@gmail.com ORCID: 0000-0001-5421-802X
Introducción: El oxígeno singulete es una especie reactiva que se obtiene mediante transferencia energética usando un fotosensibilizador. Su cuantificación directa requiere de instrumentación costosa, por lo cual es necesario recurrir a métodos indirectos que tengan suficiente selectividad y bajo costo. Estos procedimientos se basan en la interceptación química del oxígeno singulete produciendo una especie que se pueda detectar por métodos analíticos convencionales. En este artículo se describe la utilización del 9-[(E)-2-feniletenil] antraceno 1 (PEA) y del 9-[(E)-2-(naftalen-2-il) etenil]antraceno 2 (NEA), como alternativas viables y económicas para la cuantificación indirecta del oxígeno singulete, en medios acuosos. Su ventaja radica en la fácil detección de la desactivación de su fluorescencia una vez son oxidados por el oxígeno singulete. Materiales y Métodos: Los compuestos se sintetizaron y caracterizaron siguiendo procedimientos previamente reportados. Su capacidad para atrapar oxígeno singulete se determinó siguiendo su oxidación fotosensibilizada en solución de H2O/THF y en parásitos de Leishmania tarentolae, empleando azul de metileno o rosa bengala como fotosensibilizadores. Las muestras experimentales se iluminaron con una lámpara de emisión de luz visible, y se utilizaron métodos espectroscópicos (absorción UV-Vis, fluorescencia, RMN-1H) y espectrometría de masas para monitorear el atrapamiento y fotooxidación. Resultados y Discusión: Las pruebas espectroscópicas demostraron la capacidad que tienen los compuestos PEA 1 y NEA 2 para atrapar oxígeno singulete en solución acuosa y dentro de parásitos de L. tarentolae. Estudios de viabilidad parasitaria demuestran que PEA 1 es citotóxico en la oscuridad y cuando los cultivos son expuestos a la luz, mientras que NEA 2 no es citotóxico en la oscuridad, pero sí lo es cuando el cultivo es expuesto a la luz. En conclusión, los compuestos estudiados pueden servir como sondas para detectar y medir la producción de oxígeno singulete en medio acuoso y potencialmente en cultivos celulares, aunque es recomendable evaluar su actividad citotóxica en la oscuridad y bajo iluminación en estos casos.
Palabras clave: 9-[(E)-2-feniletenil]antraceno, 9-[(E)-2-(naftalen-2-il)etenil]antraceno, trampas para oxígeno singulete, efecto fotodinámico, Leishmania tarentolae
Introduction: Singlet oxygen is a reactive species obtained via energy transfer using a photosensitizer. Its direct quantification requires expensive instrumentation, so it is necessary to use indirect methods having sufficient selectivity and low cost. These procedures are based on the chemical interception of singlet oxygen producing a species that can be detected using conventional analytical methods. This article describes the utilization of 9-[(E)-2-phenylethenyl]anthracene 1 (PEA) and 9-[(E)-2-(naphtalen-2-yl)ethenyl]anthracene 2 (NEA) as suitable and economic alternatives for the indirect quantification of singlet oxygen in aqueous media. Their advantage is the easy detection of their fluorescence once they are oxidized by singlet oxygen. Materials and Methods: Compounds were synthesized and characterized following procedures previously reported. Their capacity to trap singlet oxygen was determined by monitoring their photosensitized oxidation in either a H2O/THF solution or within Leishmania tarentolae parasites, utilizing methylene blue or rose bengal as photosensitizers. Experimental samples were illuminated with a lamp emitting visible light, while spectroscopical techniques (absorption, fluorescence, 1 H-NMR) and mass spectrometry were used to monitor trapping and photooxidation. Results and Discussion: Spectroscopical evidence demonstrates that both PEA 1 and NEA 2 are capable of trapping singlet oxygen in both aqueous media and within L. tarentolae parasites. Viability studies demonstrate that PEA 1 is cytotoxic in the dark and when parasite cultures were exposed to light, while NEA 2 does not show dark cytotoxicity, but is toxic when cultures were exposed to light. It can be concluded that both compounds under study may be utilized as probes to detect and quantify the production of singlet oxygen in aqueous media and potentially in cell cultures, although it is recommended to evaluate their cytotoxic activity both in the dark and upon light exposure in these cases.
Key words: 9-[(E)-2-phenylethenyl]anthracene, 9-[(E)-2-(naphthalen-2-yl)ethenyl]anthracene, singlet oxygen traps, photodynamic effect, Leishmania tarentolae.
Photodynamic therapy (PDT) is based on methods to promote cell apoptosis of diseased tissues or pathogens (bacteria, protozoan parasites or fungi) by the photosensitized production of singlet oxygen ¹O2 (¹▲g), using visible light (1-4). Singlet oxygen, a reactive form of oxygen, is generated by energy transfer from a photosensitizer to molecular oxygen O2, thus the efficiency of PDT partially relies on the photosensitizer's ability to produce ¹O2 (¹▲g) in intracellular environments. PDT has been proven to be effective for the treatment of cancer (3-5), photo inactivation of viruses from blood derivatives (6, 7), and the treatment of ocular illnesses (3, 8). The production of intracellular ¹O2 (¹▲g) can be detected and measured using near infrared emission (9), but this methodology requires expensive instrumentation as it demands high levels of sensitivity. Alternatively, indirect detection and quantification methods are available (10). This involves chemical trapping of ¹O2 (¹▲g) by certain compounds giving rise to photoactive adducts that are detectable with conventional spectroscopic or chromatographic procedures (11-17). Unfortunately, some methods may not selective enough for singlet oxygen only, or they lack the required specificity for intracellular environments, so it is important to develop cheap, selective, indirect methodologies to detect and measure singlet oxygen. One of the methodologies widely used involves the chemical reaction of singlet oxygen with molecules in order to yield a primary specific product of the oxidation. Ideally the trapping compound should meet certain conditions such as being water soluble, highly reactive with singlet oxygen, and unreactive with other oxygen species such as triplet oxygen (³O2), superoxide radical anion (O2•-) or hydrogen peroxide (H2O2) (18). It is, however, difficult to totally avoid the oxidation of organic compounds by oxidants such as ozone (O3), hydroxyl radical (OH•), peroxyacids (RCOOOH), or peroxide and alkoxy radicals (ROO• and RO•). It is therefore difficult to design of a trap that can be activated only by the reaction with singlet oxygen.
This paper describes our work with 9-[(E)-2-phenylethenyl]anthracene, PEA 1, and 9-[(E)-2-(naphtalen-2-yl)ethenyl]anthracene, NEA 2, as potential intracellular singlet oxygen traps. These compounds are suitable as they contain the anthracene moiety, which is prone to oxidation by singlet oxygen via a selective [4p+2p] addition of this species to the central ring of the anthracene. Furthermore, the compounds are easy to synthesize at low cost, they are highly fluorescent and show moderate solubility in aqueous media, which should facilitate their detection in intracellular environments. Photosensitized oxidation of the compounds in aqueous phase was carried out as well as biological tests using the parasite Leishmania tarentolae. This has been done to evaluate the potential cytotoxicity of the compounds towards Leishmania sp. parasites that cause cutaneous leishmaniasis, a tropical disease that affects millions around the world. The photodynamic treatment of cutaneous leishmaniasis has been explored as a therapeutic option (19, 20) and thus the L. tarentolae in vitro model was used to evaluate the utilization of PEA 1 and NEA 2 as singlet oxygen traps and their toxicity towards these parasites.
Commercially available solvents and the photosensitizers rose bengal disodium salt (RB) and methylene blue (MB), were used without any additional treatment. Trap compounds PEA 1 and NEA 2were synthesized starting from commercially available materials as described below, purified by column chromatography, and characterized by spectroscopic methods.
Synthesis and characterization of 9-[(E)-2-phenylethenyl]anthracene, PEA 1 and 9-[(E)-2-naphthylethenyl]anthracene, NEA 2. Commercially available starting materials and solvents were used without any additional treatment. PEA 1 and NEA 2were prepared by the Wittig reaction starting from benzyl bromide (reagent grade, Alfa Aesar) and 9-anthracenecarboxaldehyde (reagent grade, Sigma-Aldrich), and from 2-naphthylmethyl bromide (reagent grade, Alfa Aesar) and 9-anthracenecarboxaldehyde, respectively, in dichloromethane (Mallinckrodt Chemicals) following standard literature procedures (21, 22). The products were fully characterized by 1H and 13C NMR (Bruker Ultrashield 400, tuned at 400 MHz for 1H and 100 Hz for 13C) and ESI-MS (Agilent Series 1100 MSD, 50-3,000 m/z range). Signals of the products matched the spectroscopic data reported in the literature for the respective compounds (23).
Study of the photosensitized oxidation of 1 and 2 by UV spectroscopy. A 0.1 mM standard solution of either trap compound PEA1 or NEA 2was prepared in a binary 1:1 THF-water mixture with or without 0.01 mM of the photosensitizer RB and their respective UV emission and absorption spectra were recorded. Aliquots of the standard solutions were irradiated with a visible light lamp (halogen or xenon, 75 W) for the specified periods of time. The same protocol was followed when MB was used as a photosensitizer. Absorption spectra were recorded using a Unicam UV-500 instrument, while emission spectra were recorded using a Perkin Elmer LS 55 luminescence spectrometer.
Singlet oxygen generation. Using clean quartz cells (1 cm in length), 1 mL aliquots of each solution containing trap and photosensitizer as described above were exposed to visible light during consecutive periods of 5 or 10 minutes for a total exposure time of 75 minutes. After each period of exposure to light, emission and absorption spectra were recorded. For comparison, some of the samples were purged with nitrogen and kept under nitrogen atmosphere during irradiation, and then the respective emission and absorption spectra were also recorded to evaluate effects of molecular oxygen.
Study of photooxidation by NMR spectroscopy. A 400 MHz FT-NMR, Bruker instrument was used to monitor photooxidation of trap compounds PEA 1 or NEA 2. The solutions were prepared in a binary D2O/THF-d8 mixture and the NMR tubes were sealed. 1H-NMR spectra were recorded for a standard 1 mM trap solution containing rose bengal (0.2 mM) both before and after visible light irradiation with a 75 W Xenon lamp (PTI Technologies, Model A-1010B) during 5 hours for PEA 1 and 7 hours for NEA 2.
Study of photooxidation by Mass Spectrometry (MS). A quadrupole mass spectrometer equipped with an electrospray ionization source (ESI-MS, Agilent 1100 MSD) was also used to monitor photooxidation of trap compounds PEA 1 or NEA 2 before and after irradiation. Acetonitrile, acidified with 0.1% TFA, was used as the eluent in the ionization source.
Assessment of in vitro cellular viability of L. tarentolae promastigotes during photooxidation of PEA 1 or NEA 2. A comparative study of population growth in vitro of promastigotes parasites was carried out with and without visible light exposure by using the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2Htetrazolium (MTS, Promega) cell viability probe. Parasites (ATCC #30143) were cultured in sterile brain heart infusion (BHI) medium with heme at 26 °C as reported by Morgenthaler et al. (20). Trap compounds PEA 1 or NEA 2 were dissolved in dimethyl sulfoxide (DMSO) and a volume of each solution was added during the optimal growing phase to yield a final concentration of 10 uM trap and 1% DMSO. Parasites (100 uL) were placed into polypropylene 96 well plates (Falcon) and analyses of cellular viability using the MTS reagent were carried out on days one and two post incubation under the following conditions: (i) cultures with no light and no additions; (ii) cultures plus DMSO (1% v/v) with no light; (iii) cultures plus DMSO in the presence of light; (iv) cultures plus DMSO and 10 uM PEA 1 with no light; (v) cultures plus DMSO and 10 uM PEA 1 in the presence of light; (vi) cultures plus DMSO and 10 uM NEA 2 with no light; (vii) cultures plus DMSO and 10 uM NEA 2 in the presence of light. Light absorption (at 550 nm) by the formazan resulting from MTS metabolism by live parasites was used to monitor viability using a Bio-Rad microplate reader. In order to demonstrate the occurrence of a photodynamic effect, cell samples were exposed to visible light irradiation (from a fluorescent lamp, 30 W, 350 to 750 nm at a distance of 20 cm) during two hours each day for the two days following compound addition. The cells that were incubated with no light but with trap compounds PEA 1 or NEA 2 were kept in a dark environment. The percent of parasite viability was calculated as the mean absorbance of the experimental cells (with added PEA 1 or NEA 2 in DMSO with or without light treatment) divided by the mean absorbance of the appropriate same day control cells (with DMSO but no added PEA 1 or NEA 2 and with or without light treatment) times 100. All determinations were done in triplicate and values reported as % parasite viability relative to the appropriate control (to which the test compounds were not added).The intracellular accumulation of the trap compounds PEA 1 or NEA 2 by Leishmania promastigotes was monitored using an inverted microscope (Leica 2), in the fluorescence mode, with violet/blue excitation derived from a filtered-mercury lamp. In order to observe the compounds' photoemission inside the parasites a CCD camera was used.
Reactivity of the trap compounds, PEA 1 or NEA 2, with singlet oxygen inside Leishmania parasites was also examined by emission spectroscopy. Parasites were cultured four days before incubation with a 1.0 uM PEA 1 solution for 15 minutes or a 1.0 uM NEA 2 solution for an hour. Then a 2 mL aliquot of the Leishmania culture was centrifuged in a tabletop Eppendorf 5415C instrument at 4000 rpm for one minute and the supernatant was discarded. Cells were resuspended in sterile BHI medium (1 mL) without heme and this process was repeated twice to remove exogenous compounds.
Following the last resuspension in fresh hemefree medium, the cells were transferred to a quartz cell and the emission spectra were obtained while compared with a blank of hemefree BHI medium. Spectra were recorded for cells with or without visible light exposure.
In this study, known compounds 9-[(E)-2-phenylethenyl]anthracene PEA 1, and 9-[(E)-2-(2-naphthalenyl)ethenyl]anthracene NEA 2, (Scheme 1), were used for singlet oxygen ¹O2 (¹▲g) trapping and their biological photodynamic effect on cellular cultures of L. tarentolae promastigotes was also examined.
The photophysical properties of the compounds have been previously determined (24-26). The emission band near 500 nm has been assigned to fluorescence, and quantum yields have been determined to be about 0.5 for PEA 1 and about 0.6 for NEA 2 in acetonitrile. Compounds have been previously shown to be stable towards E to Z isomerization with quantum yields < 0.01 in acetonitrile (24).
Chemical behavior of PEA 1 as a trap for singlet oxygen. The experiments were carried out in 1.0 mM standard solutions of PEA 1 in a binary 1:1 H2O/THF solvent system. The action of a photosensitizer was examined with 0.1 mM methylene blue, MB, or 0.1 mM rose bengal disodium salt, RB. Figure 1 shows the results of photooxidation of PEA 1 with and without MB in the presence of oxygen or in an oxygendepleted environment, monitored by emission and absorption spectroscopy, while Figure 2 shows the results of the analogous experiments using RB instead of MB as photosensitizer.
As illustrated in Figure 1(a), in the absence of a photosensitizer, the emission spectrum of PEA 1 exhibited a 400-650 nm band with a lmax at 470 nm that was not changed upon illumination during 15 minutes. Also, a comparison of the pre- and post-illumination emission spectra of PEA 1 in the presence of MB, but in an oxygenpoor environment, Figure 1(b), revealed that the characteristic 400-650 nm band was not changed after 15 min or 30 min periods of illumination. Likewise, the absorption spectrum of PEA 1, characterized by a 330-440 nm band with a lmax at 390 nm, was unchanged even in the presence of both MB and oxygen when the solution was not illuminated, Figure 1(c). These experiments indicate that PEA 1 was not bleached when a photosensitizer was not present whether oxygen was present or depleted. In clear contrast, as evidenced in Figure 1(d), emission spectra of PEA 1 in the presence of MB and oxygen monitored at 10, 20, 30, 40, 45 and 60 minutes of light exposure indicates the decrease in the amount of compound. This result is consistent with an absorbance decrease in the characteristic 330-440 nm band of the absorption spectrum of PEA 1, Figure 1(e). These results suggest that in the presence of oxygen, PEA 1 is photooxidized with the action of MB as photosensitizer. Moreover, this is confirmed with a plot that illustrates the trend of the absorbance of PEA 1 at lmax = 390 nm as a function of illumination time in the presence of both oxygen and the photosensitizer, monitored at 5 min-intervals, revealing an almost linear behavior, Figure 1(f). It is important to note that while the absorbance of PEA 1 steadily decreased, the respective 550-700 nm band characteristic of MB was not changed, Figure 1(e). This indicates that the photosensitizer is not sensitive to light and singlet oxygen during the photooxidation of PEA 1.

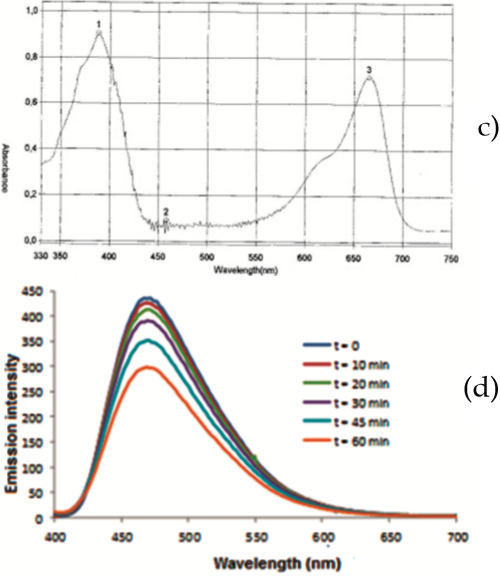


The effect of RB as photosensitizer for the photooxidation of PEA 1was also examined by emission and absorption spectroscopy in solution (1.0 mM PEA 1 in 1:1 H2O/THF and 0.1 mM RB) and NMR and ESI-MS (1.5 mM PEA 1 in 1:1 D2O/THF-d8 and 0.18 mM RB). Samples analyzed by UV-Vis spectroscopy were illuminated for up to 35 minutes, while those analyzed by NMR and ESI-MS were exposed to light for 425 minutes. Results are shown in Figure 2.
As illustrated in Figure 2, illumination of PEA 1in the presence of RB and oxygen clearly promoted changes in its emission and absorption spectra as well as its proton NMR and ESI-MS patterns. In fact, the intensity of the 400-650 nm band of the emission spectrum decreased as the sample was being illuminated, Figure 2(a). This result agrees with a continuous reduction of the absorbance at 390 nm during the illumination period monitored at 5 min intervals, Figure 2(b). The results strongly indicate that photooxidation of PEA 1 occurs by exposure to light in the presence of oxygen by action of either photosensitizer (MB or RB) tested. However, unlike MB, RB undergoes photobleaching, as evidenced by a decrease in its 500-600 nm band with time of light exposure, Figure 2(a).
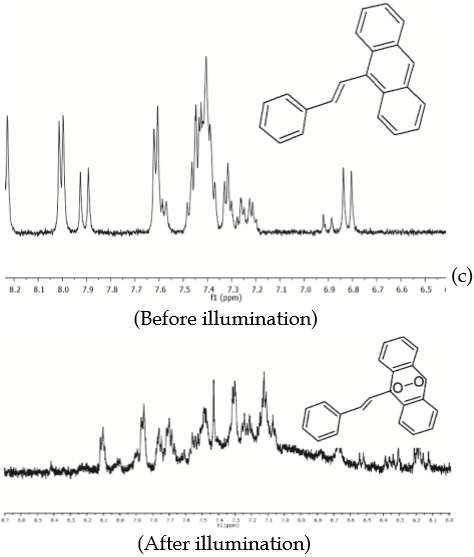


Additional evidence of photooxidation of PEA 1 by illumination in the presence of oxygen and RB is demonstrated by proton NMR and ESI-MS analyses after a 425 min-period of illumination, figures 2(c) and 2(d). PEA 1 exhibits a characteristic signal at 8.44 ppm, which is assigned to the proton attached to C10 [upper panel, Figure 2(c)]. This signal disappears completely after illumination [lower panel, Figure 2(c)], presumably due to the generation of the respective endoperoxide via a [4p+2p], Diels-Alder pathway (27), with PEA 1 behaving as a diene and ¹O2 acting as a dienophile. Furthermore, upon comparison of the ESI-MS spectra recorded before [upper panel, Figure 2(d)] and after illumination [lower panel, Figure 2(d)], it is evident that the molecular ion of PEA 1 which corresponds to fragment m/z = 281.15 amu (MH+) is changed for two fragments: one of m/z = 313.15 amu that may be assigned to the molecular ion (MH+) of the endoperoxide adduct (two oxygen atoms have been incorporated) and other fragment of m/z = 297.15 amu resulting either from the release of an oxygen atom from the endoperoxide or by addition of an oxygen atom only to PEA 1. Chemical behavior of NEA 2 as a trap for ¹O2. Attempts to use NEA 2 as a trap for ¹O2 were carried out through an analogous series of experiments as were performed for PEA 1 under similar conditions. Likewise, photooxidation of NEA 2 was studied by emission and absorption spectroscopy, pre- and post-illumination with and without photosensitizer (MB or RB), and either in the presence of oxygen or in an oxygen-depleted medium [figures 3(b) and 3(a) respectively]. Additional evidence by NMR and ESI-MS analyses is included. Results of the experiments of photooxidation of NEA 2 with MB as photosensitizer are shown in Figure 3, while Figure 4 shows the analogous results when RB is used instead of MB.
As with PEA 1, emission spectrum of NEA 2 exhibits a 400-650 nm band with a lmax = 481 nm that is not changed in an oxygen-depleted medium or in the absence of photosensitizer MB or RB, and when the mixture is not exposed to light, Figure 3(a). However, the intensity of this band is reduced upon exposure to light in the presence of oxygen and MB [Figure 3(b)] or RB [Figure 4(a)]. Furthermore, the absorption spectrum of NEA 2 exhibits a 330-440 nm band with lmax = 390 nm and, as expected, the absorbance changes upon exposure to light in the presence of oxygen and MB or RB [Figure 3(c) and Figure 4(b), respectively]. Experiments with NEA 2 and MB also demonstrated that this photosensitizer is not bleached during illumination [Figure 3(b)] whereas RB experienced photobleaching under identical conditions [Figure 4(a)].

As seen in Figure 4(c) and 4(d), NMR and ESIMS experiments with NEA 2 gave rise to about the same results as with PEA 1. The 1 H-NMR spectrum of NEA 2 exhibits a signal at 8.45 ppm (s) that is assigned to the proton attached to C10, which is no longer present after a 300 min-period of exposure to visible light, presumably because it has moved up field from the aromatic region due to the addition of oxygen. Comparison of the ESI-MS spectra that were recorded before and after exposure to light indicates that ¹O2 has been incorporated to the molecule [Figure 4(d)]. The ESI-MS molecular ion is changed from m/z = 331.15 amu (corresponds to NEA 2 before illumination) to two ions of m/z = 363.15 amu and m/z = 347.15 amu which correspond to adducts with two and one oxygen atoms, respectively.

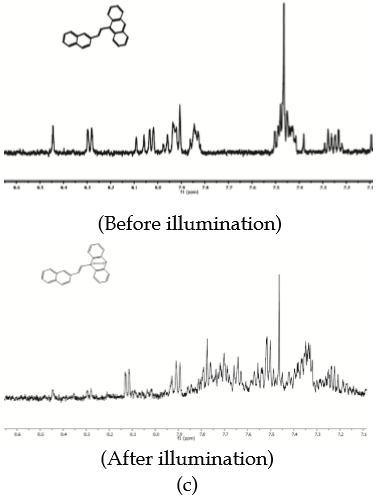
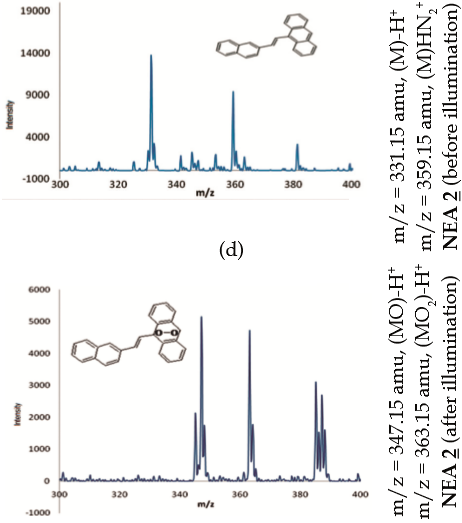

Study of the photodynamic effect of PEA 1 and NEA 2 on L. tarentolae promastigotes. Since PEA 1 and NEA 2 were demonstrated to behave as trap compounds for ¹O2, tests were carried out to assess their ability to penetrate L. tarentolae promastigotes, a parasitic protozoan that infects reptiles, which are useful for pre-screening tests for potential photodynamic drugs (28).
L. tarentolae parasites were incubated in the presence of either compound then washed to remove remaining compound in the medium. The results clearly demonstrate that both PEA 1 and NEA 2 can be found inside the parasites (Figure 5). These compounds appear to localize in non-identified cellular regions. It was found that PEA 1 gets inside Leishmania cells within 15 minutes while the NEA 2 uptake required about an hour.
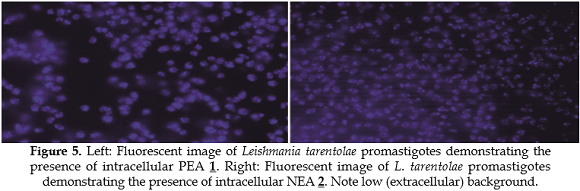
To determine if PEA 1 and NEA 2 exhibit cytotoxic activity, their antileishmanial activity was evaluated using a standard cell viability methodology (20,27). The results are shown in Figure 6.
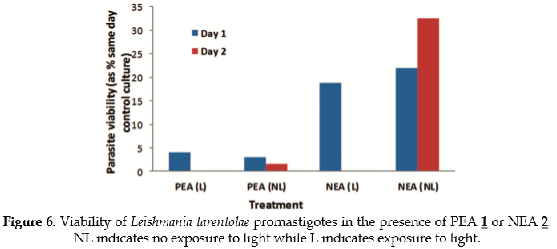
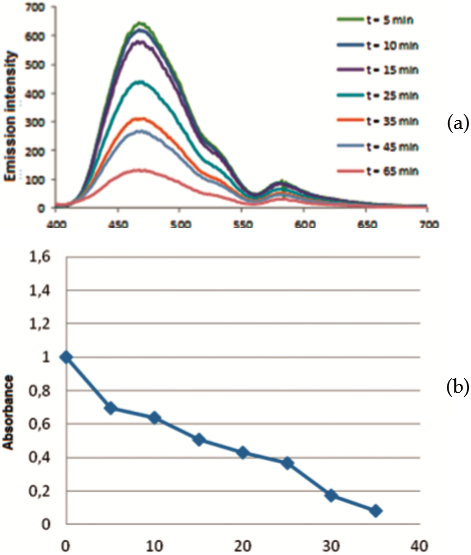
Both compounds at 10 uM concentration are efficient at inhibiting the growth of Leishmania promastigotes in vitro. Figure 6 shows parasite viability as the relative percentage of surviving parasites with respect to control cultures with no added compounds. It is clear that PEA 1 is an antileishmanial substance even in the dark, and that cells do not grow in the culture especially after light treatment. On the other hand, addition of NEA 2 does not exhibit such a strong antileishmanial effect in the dark or even after the first day of exposure to visible light; however by day 2 NEA 2 does exhibit photodynamic inhibition of parasite growth since no viable parasites are detected. This is in contrast to the growth, relative to control cells, seen on day 2 without light exposure. The contrasting photodynamic effect between day 1 and day 2 when comparing both compounds may be due to the dark toxicity of PEA 1. The antileishmanial activity of both PEA 1 and NEA 2 at day 1 is very similar, regardless of whether the parasites were exposed to light or not. However, exposure to light for a second day enhances the activity of the compounds. Both compounds are likely to produce reactive oxygen species, including singlet oxygen within the parasites. Indeed, PEA 1 and NEA 2 intracellular accumulation by parasites was examined by measuring their fluorescence directly in samples of cultures of parasites incubated with either compound in the absence of photosensitizer, and then washed to remove exogenous compounds. Cells were resuspended in heme free medium. In these experiments, emission intensity of intracellular PEA 1 and NEA 2 clearly dropped as a function of time of light exposure as shown in Figure 7. It is clear that singlet oxygen has been trapped by the compounds even in the absence of a photosensitizer, which implies that the compounds generate and trap this species upon absorption of violet light (around 400 nm).

These results strongly suggest that both compounds PEA 1 and NEA 2 are able to generate ¹O2 and be self-oxidized inside the parasite. It is known that NEA 2 photosensitizes the production of singlet oxygen in air saturated solutions of acetonitrile (quantum yield 0.38) and methylcyclohexane (quantum yield 0.52) upon exposure to 355 nm light (24). The quantum yield for singlet oxygen production by PEA 1 under similar conditions is not known, but Shin and coworkers (24) report triplet state yields for both compounds. The triplet state yield of PEA 1 is reported to be larger (0.27) than for NEA 2 (0.20) in oxygen-saturated acetonitrile solutions.
If one assumes that the production of singlet oxygen is proportional to the amount of excited triplet state, then PEA 1 would exhibit a larger singlet oxygen yield than NEA 2. It has been shown by Shin et al. (24) that singlet oxygen production is also mediated by the excited singlet state of these compounds. Our results imply that PEA 1 is more effective than NEA 2 at producing singlet oxygen and self-oxidizing inside the parasite and does not require additional light exposure, which seems to agree with what would be expected based on the photophysical properties of the compounds. These demonstrate that a photodynamic effect is largely responsible for the anti-leishmanial activity of PEA 1 or NEA 2, although the activity of PEA 1 in the dark must be attributed to a different additional mechanism of action. A plausible explanation may involve the action of an endoperoxide derivative, which could be obtained by cellular oxidation reactions. Some endoperoxides like artemisinin are well-known antimalarials (29), which act by formation of a radical species that result from endoperoxide cleavage by iron followed by alkylation of parasite's key proteins, which renders them inactive. It is not known, however, why the endoperoxide from PEA 1 may be active while that from NEA 2 may not, but it is plausible that NEA 2 does not distribute as well as PEA 1 in critical organelles within the parasites.
In order to demonstrate the photodynamic effect that is caused by RB as a photosensitizer, the spectral range that excites trap compounds PEA 1 or NEA 2 was blocked using a light filter (long pass above 500 nm), thus avoiding selfoxidation. The respective results are presented in Figure 8.
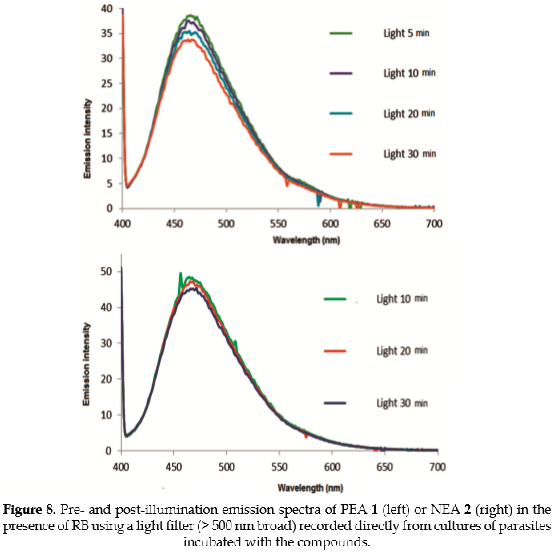
The results suggest that the photosensitizer RB is not very effective inside the cell at reducing the emission of trap compounds PEA 1 or NEA 2. This result may be rationalized in terms of the effectiveness of energy transfer inside the parasite, which seems to be diminished because of various factors such as: (i) the lack of spatial proximity inside the cellular environment; or (ii) extinction of ¹O2 that is generated by RB exerting a photodynamic effect in other intracellular species, such as protein, lipid, or nucleic acids, prior to its collision with the trap compound.
In conclusion, experiments on photosensitized oxidation were carried out using the synthetic probes PEA 1 or NEA 2. The results strongly suggest that these synthetic probes are able to trap singlet oxygen ¹O2 (¹▲g) in aqueous systems with significant specificity and sensitivity. These ¹O2 trap compounds react, presumably via a [4p+2p] cycloaddition pathway, giving rise to the respective endoperoxides. PEA 1 or NEA 2 solutions exhibit a characteristic intense absorptivity in the UV-Vis 330-440 nm region and, upon reaction under the proper conditions for a photosensitized oxidation, evident changes in the fluorescence intensity are observed in the 400-650 nm region. These probes were found to be photochemically stable under the experimental conditions utilized here. Additional proof that oxidation occurs in the presence of methylene blue or rose bengal photosensitizers were obtained by NMR spectroscopy and ESI-MS spectrometry analysis.
Experiments on cellular viability of L. tarentolae promastigotes incubated in the presence of PEA 1 or NEA 2 solutions were also performed, and demonstrated efficient transport into the parasites. PEA 1 proved to be an effective antileishmanial compound reducing cell viability on day 1 by about 95% relative to control cells.
This inhibition was evident both with and without exposure to light with a greater inhibitory effect on day 1 following light treatment. NEA 2 exhibited less anti-leishmanial activity in dark (about 70% reduced viability on day 2) but evidenced a significant photodynamic effect at inducing parasite mortality following exposure of L. tarentolae cultures to light with an apparent 100% reduction of cell viability on day 2.
It is concluded that these trap compounds potentially represent an inexpensive tool for detection and monitoring of singlet oxygen ¹O2 (¹▲g) in aqueous and cellular environments. They may be useful for related research and studies on cellular apoptosis related to photodynamic therapy.
Authors want to acknowledge the Department of Chemistry at Illinois State University for financial support. CAM, LAR, RO also want to acknowledge the Vicerrectoría de Investigaciones y Postgrados at Universidad de Caldas for financial support. DLC thanks financial support of a Program of Excellence award from the College of Arts and Sciences at Illinois State University.
1. Pass HI. Photodynamic therapy in oncology: mechanisms and clinical use. J. Natl. Cancer Inst. 1993; 85:443-456. [ Links ]
2. Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl. Cancer Inst. 1998; 90:889-905. [ Links ]
3. Photodynamic Therapy of Neoplastic Disease. Volume I, Kessel D, ed. Boca Raton, Florida: CRC Press; 1990. [ Links ]
4. Weishaupf KR, Gomer CJ, Dougherty TJ. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976; 36:2326-2329. [ Links ]
5. Allison RR, Moghissi K. Oncologic photodynamic therapy: clinical strategies that modulate mechanisms of action. Photodiagnosis Photodyn. Ther. 2013; 10:331-341. [ Links ]
6. North J, Neyndorff H, Levy JG. Photosensitizers as virucidal agents. J. Photochem. Photobiol. B. 1993; 17:99-108. [ Links ]
7. O'Brien JM, Gaffney DK, Wang TP, Sieber F. Merocyanine 540-sensitized photoinactivation of enveloped viruses in blood products: site and mechanism of phototoxicity. Blood 1992; 80:277-285. [ Links ]
8. Mennel S, Barbazetto I, Meyer CH, Peter S, Stur M. Ocular photodynamic therapy-standard applications and new indications. Part 2. Review of the literature and personal experience. Ophthalmologica 2007; 221:282-291. [ Links ]
9. Niedre M, Patterson MS, Wilson BC. Direct near-infrared luminescence detection of singlet oxygen generated by photodynamic therapy in cells in vitro and tissues in vivo. Photochem. Photobiol. 2002; 75:382-391. [ Links ]
10. Halliwell B, Whiteman M. Measuring reactive oxygen species and oxidative damage in vivo and in cell culture: how you should do it and what do the results mean. Brit. J. Pharmacol. 2004; 142:231-255. [ Links ]
11. Nardello V, Aubry J-M. Measurement of photogenerated singlet oxygen in aqueous media. Meth. Enzymol. 2000; 319:50-58. [ Links ]
12. Umezawa N, Tanaka K, Urano Y, Kikuchi K, Higuchi T, Nagano T. Novel fluorescent probes for singlet oxygen. Angew. Chem., Int. Ed. 1999; 38:2899-2901. [ Links ]
13. Tanaka K, Miura T, Umezawa N, Urano Y, Kikuchi K, Higuchi T, Nagano T. Rational design of fluorescein-based fluorescence probes. Mechanism-based design of a maximum fluorescence probe for singlet oxygen. J. Am. Chem. Soc. 2001; 123:2530-2536. [ Links ]
14. Song B, Wang G, Tan M, Yuan J. Synthesis and time-resolved fluorometric application of a europium chelate-based phosphorescence probe specific for singlet oxygen. New J. Chem. 2005; 29:1431-1438. [ Links ]
15. Song B, Wang G, Yuan J. A new europium chelate-based phosphorescence probe specific for singlet oxygen. Chem. Commun. 2005; 28:3553-3555. [ Links ]
16. Song B, Wang G, Tan M, Yuan J. A europium (III) complex as an efficient singlet oxygen luminescence probe. J. Am. Chem. Soc. 2006; 128:13442-13450. [ Links ]
17. Dai Z, Tian L, Xiao Y, Ye Z, Zhanga R, Yuan J. A cell-membrane-permeable europium complex as an efficient luminescent probe for singlet oxygen. J. Mater. Chem. B 2013; 1:924-927. [ Links ]
18. Gollmer A, Arnbjerg J, Blaikie FH, Pedersen BW, Breitenbach T, Daasbjerg K, et al. Singlet Oxygen Sensor Green: photochemical behavior in solution and in a mammalian cell. Photochem. Photobiol. 2011; 87:671-679. [ Links ]
19. Taylor VM, Cedeño DL, Muñoz DL, Jones MA, Lash TD, Young AM, et al. In vitro and in vivo studies of the utility of dimethyl and diethyl carbaporphyrin ketals in treatment of cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2011; 55:4755-4764. [ Links ]
20. Morgenthaler JB, Peters SJ, Cedeño DL, Constantino MH, Edwards KA, Kamowski EM, et al. Carbaporphyrin ketals as potential agents for a new photodynamic therapy treatment of leishmaniasis. Bioorg. Med. Chem. 2008; 16:7033-7038. [ Links ]
21. Becker HD, Andersson K. On the relationship between molecular geometry and excited-state properties of 9-anthrylalkenes. J. Org. Chem. 1983; 8:4542-4549. [ Links ]
22. Bhattacharyya K, Chattopadhyay SK, Baral-Tosh S, Das PK. Excited state properties of trans-(9-anthryl) ethylenes. Effects of geometric distortion about single bond. J. Phys. Chem. 1986; 9:2646-2651. [ Links ]
23. Jaworek C, Lacobucci S. Wittig Reaction: The Synthesis of trans-9-(2 phenylethenyl)anthracene Revisited. J. Chem. Educ. 2002; 79(1):111p. [ Links ]
24. Shin EJ, Stackow R, Foote CS. Excited state properties of some 1-(9-anthryl)-2-naphthylethene and 1-(9-anthryl)-2-quinolylethene derivatives. Phys. Chem. Chem. Phys. 2002; 4:5088-5095. [ Links ]
25. Bartocci G, Masetti F, Mazzucato U, Spalletti A, Orlandi G, Poggi G. A photophysical and theoretical study of styrylanthracenes. J. Chem. Soc., Faraday Trans. 2. 1988; 84:385-399. [ Links ]
26. Latterini L, Elisei F, Aloisi GG, Rodgers MAJ. Dynamics of the singlet excited states of diarylethenes in the presence of charge-transfer interactions. A picosecond laser flash photolysis study. J. Phys. Chem. A 1997; 101:9870-9876. [ Links ]
27. Corey EJ, Taylor WC. A study of the peroxidation of organic compounds by externally generated singlet oxygen molecules. J. Am. Chem. Soc. 1964; 86:3881-3882. [ Links ]
28. Taylor VM, Muñoz DL, Cedeño DL, Vélez ID, Jones MA, Robledo SM. Leishmania tarentolae: Utility as an in vitro model for screening of antileishmanial agents. Exp. Parasitol. 2010; 126:471-475. [ Links ]
29. Meshnick SR. The mode of action of antimalarial endoperoxides. Trans. Roy. Soc. Trop. Med. Hyg. 1994; 88(Sup.1):31-32. [ Links ]
Como citar este artículo: Marín-Londoño CA, Brand SB, Ríos-Vásquez LA, Ocampo-Cardona R, Jones MA, Cedeño DL. 9-[(E)-2-phenylethenyl]anthracene and 9-[(E)-2-(naphthalen-2-yl)ethenyl]anthracene as traps for singlet oxygen: photosensitized oxidation and photodynamic effect on Leishmania tarentolae parasites. Revista Biosalud 2016; 15(1):25-40. DOI: 10.17151/biosa.2016.15.1.4














