Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biotecnología en el Sector Agropecuario y Agroindustrial
Print version ISSN 1692-3561
Rev.Bio.Agro vol.9 no.2 Popayán Jul.y/Dec. 2011
EFECTO DE LA BIOFERTILIZACIÓN SOBRE EL CRECIMIENTO EN MACETA DE PLANTAS DE CAÑA DE AZÚCAR (Saccharum officinarum)
EFFECT OF BIOFERTILIZATION ON THE GROWTH OF POTTED SUGARCANE PLANTS (Saccharum officinarum)
EFEITO DA BIOFERTILIZAÇÃO NO CRESCIMENTO DE PLANTAS EM VASOS açúcar de cana (Saccharum officinarum)
LILIANA SERNA-COCK1, CAMILO ARIAS-GARCÍA1, LEIDY JOHANA VALENCIA HERNANDEZ1
1 Universidad Nacional de Colombia Sede Palmira, Facultad de Ingeniería y Administración, Carrera 32 vía Candelaria, Palmira, Colombia.
Correspondencia: lsernac@palmira.unal.edu.co
Recibido para evaluación: 22/12/2010. Aprobado para publicación: 08/06/2011
ABSTRACT
The use of microorganisms as fertilizer has demonstrated beneficial effects on plant growth and is an alternative to chemical fertilizers. However, each microorganism has different beneficial effects. This study evaluated the effect of applying microorganism fertilizers, Azospirillum brasilense, Azotobacter chroccocum, and Trichoderma lignorum on the growth of potted sugarcane plants var. CC 934418. Plant growth was measured in terms of stem diameter, stem and root length, and the number of leaves and roots 15, 30, and 45 days after planting. Plant growth evidenced statistically significant differences among treatments. Microorganism fertilizers showed a positive effect on the growth of sugarcane plants, with Azospirillum brasilense and Trichoderma lignorum as the microorganisms that exercised the greatest effect on stem diameter, root systems, and plant foliation. Beneficial effects of Trichoderma lignorum on leaf growth were observed. This is a new scientific contribution since this species has not been reported as promoting plant growth.
KEYWORDS:
Saccharum, growth, azospirillum brasilense, azotobacter chroccocum, trichoderma lignorum.
RESUMEN
El uso de microorganismos como fertilizante, ha demostrado tener efectos benéficos sobre el crecimiento de plantas y son una alternativa al uso de fertilizantes guímicos, sin embargo, cada microorganismo difiere en sus efectos benéficos. En este trabajo se evaluó el efecto de la aplicación de microorganismos fertilizantes, Azospirillum brasilense, Azotobacter chroccocum y Trichoderma lignorum sobre el crecimiento en maceta de plantas de caña de azúcar variedad CC 934418. El crecimiento de las plantas se midió en términos de diámetro del tallo, longitud de tallo y raíces, y número de hojas y raíces a los 15, 30 y 45 días de la siembra. El crecimiento de las plantas mostró diferencias estadísticamente significativas entre los tratamientos. Los microorganismos fertilizantes mostraron efecto positivo sobre el crecimiento de plantas de caña de azúcar, siendo Azospirillum brasilense y Trichoderma lignorum los microorganismos gue ejercieron mayor efecto sobre el diámetro del tallo y los sistemas radical y foliar de la planta. Se observaron los efectos beneficiosos de Trichoderma lignorum sobre el crecimiento de la hoja. Este es un nuevo aporte científico, ya que esta especie no ha sido reportada como promotora de crecimiento vegetal.
PALABRAS CLAVE:
Caña de azúcar, crecimiento, azospirillum brasilense, azotobacter chroccocum, trichoderma lignorum.
RESUMO
0 uso de microrganismos como fertilizante, tem sido demonstrado gue têm efeitos benéficos no crescimento das plantas e são considerados uma alternativa ao uso de fertilizantes guímicos, no entanto, cada microrganismo possui diferentes efeitos benéficos. Neste estudo foi avaliado o efeito da aplicação de microorganismos fertilizantes, Azospirillum brasilense, Azotobacter chroccocum e Trichoderma lignorum no crescimento de cana-de-açucar da variedade CC 934418 plantadas em vasos. 0 crescimento das plantas foi medido em termos do diâmetro do caule, comprimento de caule e da raiz e número de folhas e raízes nos dias 15, 30 e 45 após a semeadura. 0 crescimento da planta mostrou diferenças significativas entre os tratamentos. Os microrganismos fertilizantes mostraram efeito positivo sobre o crescimento das plantas de cana, os microrganismos Azospirillum brasilense e Trichoderma lignorum exerceram um efeito maior em diâmetro do caule, sistema radicular e folhas da planta. Os efeitos benéficos do Trichoderma em lignorum crescimento da folha foram observadas. Esta é uma nova contribuição científica vez que esta espécie não foi relatada como a promoção de crescimento vegetal.
PALAVRAS-CHAVE:
cana-de-açucar, crescimento, Azospirillum brasilense, Azotobacter chroccocum, Trichoderma lignorum.
INTRODUCTION
Biofertilization has been used as an alternative to chemical fertilizers to increase soil fertility and crop production in sustainable agriculture [1]. Use of microorganisms beneficial to agriculture started more than 60 y ago because of their capacity to convert unavailable and nutritionally important elements into available ones, and as an alternative to increase plant resistance to adverse environments [2,3]. Among the microorganisms that have had a beneficial effect on crop growth and yield, and found to be associated to plant rhizosphere, are species of the genera: Azospirillium, Azotobacter, Acinetobacter, Alcaligenes, Arthrobacter, Bacillus, Beijerinckia, Burkholderia, Enterobacter, Erwinia, Flavobacterium, Rhizobium, Serratia, and Trichoderma [4]. These microorganisms species benefit plant development owing to the fact that they increase nitrogen absorption, phytohormone synthesis, mineral solubilization, and iron chelates. Some can also induce resistance against pests and inhibit soil pathogens through the production of antimicrobial metabolites. Furthermore, they improve soil structure and reduce erosion [5, 3, 6, 7].
Diverse studies report that inoculation with only one beneficial microorganism generally increases plant growth and decreases pathogenic agents [8, 9]. Each microorganism has different beneficial effects, the inoculation effect with Azospirillum on plant growth, for example, is due to a secretion of substances promoting plant growth, mainly indoleacetic acid producing a positive effect on root development and water and mineral catchment [10,11]. The beneficial effect of the Trichoderma spp. microorganism is that it establishes symbiotic rather than parasitic relationships with the plant increasing root growth and productivity, helping to overcome stress situations, and improving nutrient absorption [12]. Species of the Trichoderma genus are able to inhibit the growth of a variety of potentially pathogenic fungi, such as Botrytis cinerea, Mucor piriformis, Rhizoctonia solani, Alternaria alternata, Fusarium udum, and Sclerotium cepivorum [13, 14, 15,16,17]. However, the lignorum species is not reported as having biofertilizing potential. Bacteria of the Rhizobiacea genus establish symbiotic relationships with the roots of legumes and the effectiveness of this symbiosis will depend on the capacity of the legumes to form consistent nodules in their plant tissue and nitrogen fixation necessary for plant development under stress conditions. Furthermore, these bacteria stimulate phytohormone production, such as cytokinins, auxins, and gibberelins that are responsible for plant growth or elongation of the cell wall. In turn, the plant contributes all the necessary organic compounds for the growth of the microorganism [18,19,20]. In spite of the benefits that these microorganisms give to plants, few real applications are reported [1]. In the tomato (Lycopersicon esculentum Mill), inoculation with rhizobacteria exerted a positive effect on quality, specifically in size and texture [21]. Other authors have reported that they were able to increase growth and yield in apricots by using rhizosphere microorganisms [22], peanuts [23], and cacti [24], whereas Kizilkaya [25], found that strains of Azotobacter chroococcum exerted a positive effect on nitrogen yield and concentration in wheat.
Few studies have been found about biofertilizer use for sugarcane, in spite of the fact that asymbiotic nitrogen fixed by bacteria replaces 60% of the nitrogen needed by this cultivar (corresponding to kg N ha-1) and it is harvested in more than 90 countries worldwide [26,27, 28]. Mirza et al. [29], inoculated sugarcane seedlings micropropagated with strains of Enterobacter sp and achieved beneficial effects on growth. These authors indicated the potential of these strains as biofertilizers in sugarcane. Suman et al. [30], found substantial genetic diversity in some strains of Acetobacter for future research as microorganisms promoting plant growth, and suggested that strains of Acetobacter and Azospirillum can be used as the basic source for the development of sugarcane biofertilizers. Stamford et al. [31], evaluated the effectiveness of a biofertilizer on sugarcane yield and its effects on some chemical attributes of a soil with low P and K availability, and considered that biofertilizers are a potential source for use in sugarcane.
Sugarcane fertilization consists in contributions of nutrients of the Nitrogen-Phosphorus-Potassium (NPK) type with the actual recommended dose of 250, 75, and 190 kg ha-1, respectively, for each component. However, other authors recommend additional doses of NPK in clay soils (463, 261, and 226 kg/ha, respectively) whereas partially saline clay soils also require additional doses of NPK, 151, 121, and 35 kg ha-1, respectively [32]. The indiscriminate use of chemical compounds for crop fertilization entails removal of nutrients and secondary micronutrients present in the soil, extracted from one harvest to another and without returning in an organic form. Due to this problem, it is necessary to apply sustainable agriculture through beneficial microorganisms or biofertilizers which not only compensate nutrient deficiencies, but rather increase crop productivity and plant nutrient assimilation efficiency [33]. Consequently, this study evaluated the effect of applying Azospirillum brasilense, Azotobacter chroccocum, and Trichoderma lignorum in sugarcane plant var. CC 934418 on stem diameter, stem and root length, and number of leaves and roots, and they were compared with sugarcane plants grown in a nutrient-rich substrate.
METHOD
Thirty-six certified sugarcane plants var. CC 934418 were obtained from Ingenio del Cauca Hacienda, Zanjón Rico, suerte 2, Miranda (Cauca, Colombia). Plants were potted in a nutrient-rich substrate, composition shown in Table 1.
Subsequently, 2 d after planting, 27 sugarcane plants were inoculated separately with 10 mL aqueous solutions of Azospirillum brasilense, Azotobacter chroccocum, and Trichoderma lignorum in concentrations of 2.5x107CFU mL-1. Strains were obtained from the microbial seed bank of the Biocontrol company and certified in the soil microbiology laboratory of the Universidad Nacional de Colombia, Bogotá campus, and in the Center for Microbiological Research of the Universidad de los Andes in Bogotá (CIMIC), Colombia.
After 15, 30, and 45 d of the inoculation, random samples were taken to analyze the inoculated plants for each microorganism and control. Subsequently, plants were washed in a water solution with 10% sodium hypochlorite, and measurements taken of stem diameter, stem and root length, and number of leaves and roots. Stem diameter was calculated with a Vernier caliper, stem and root length with a flexometer scaled 0 to 3 m, and the number of leaves and roots was counted manually. Experiments were carried out with three replicates and a completely random 4x3 factorial design as follows:
The inoculant type factor had four levels, Azospirillum brasilense, Azotobacter chroccocum, and Trichoderma lignorum, as well as a non-inoculated treatment used as the control.
Plant development time factor had three levels: development at 15, 30, and 45 d after inoculation.
The response variable was plant development 15, 30, and 45 d after inoculation with each microorganism measured for stem diameter, stem and root length, and number of leaves and roots.
Results were analyzed with ANOVA and multiple mean tests by the minimum significant difference method (MSD) adjusted at 95% with the statistical package SAS version 9.13.
RESULTS
Stem diameter and length
Figure 1 shows the variation in stem diameter for inoculation with Azospirillum brasilense, Azotobacter chroccocum, and Trichoderma lignorum in sugarcane plants var. CC 934418.

ANOVA demonstrated a highly significant difference among treatments. The MSD method did not show differences between the control and treatment with Azotobacter chroccocum in the three evaluated time periods. Azospirilum brasilense and Trichoderma lignorum displayed a beneficial effect on stem diameter from the first 15 d after planting. On day 30, Azospirillum brasilense significantly increased stem diameter whereas the Azotobacter chrocooccum and Trichoderma lignorum treatments did not present statistical differences. By day 45, plants inoculated with Azospirillum brasilense and Trichoderma lignorum displayed a greater diameter (0.9 mm).
Diverse studies have reported the beneficial effects of biofertilizers on plant growth. Singh and Datta [34], obtained superior growth and grain yield in plants treated with Anabaena variabilis. Plant growth is affected by the presence of microorganism pathogens [35, 36] which become resistant to agrochemicals. The use of microorganism fertilizers decreases resistance against these pathogens and benefits their growth [35,36] .This is explained by the production of antibiotics, siderophores, and antagonist substances which acts as biological control [37]. The beneficial effect on stem diameter was observed in this study, although the production of the aforementioned substances was not quantified. Given the agrobiotechnological potential found in this study, identification and quantification of the named substances could be considered as the objective of a future study.
Figure 2 shows the variation in stem length for inoculation with Azospirilum brasilense, Azotobacter chrococcum, and Trichoderma lignorum in sugarcane plants var. CC 934418. ANOVA demonstrated a highly significant difference among treatments. The MSD test showed differences among all treatments in the three evaluated time periods (15, 30, and 45 6). Azospirilum brasilense and Trichoderma lignorum displayed a beneficial effect on stem length from the first 15 d after planting. Azotobacter chrococcum showed an effect on stem length starting from day 30.

Azospirillum brasilense demonstrated the greatest benefit on sugarcane plant stem length, which could be a consequence of phytohormone production that stimulating nutrient absorption and subsequent plant development. Stem length of Prunus cerasifera L. clone Mr.S 2/5 plants inoculated with Azospirillum brasilense Sp245 was significantly higher than non-inoculated plants [38]. Donoso et al. [39], reported significant differences in the height and biomass of Pinus Radiata when Trichoderma harzianum was used jointly with a nutrient-rich substrate (compost). Trichoderma spp. is considered to be a plant disease control agent and it is found on the market as a biofertilizer [12, 40]. Shukla et al. [41], suggest that the beneficial effect exerted on sugarcane yield for Trichoderma sp. is due to the soil condition as regards carbon and nitrogen requirements.
Root length and number of roots
Figures 3 and 4 show the respective variations in root length and number of roots with inoculation with Azospirillum brasilense, Azotobacter chrococcum, and Trichoderma lignorum in sugarcane plants var. CC 934418.
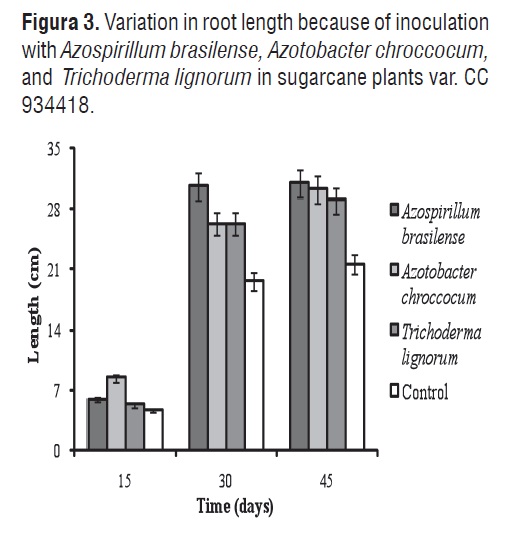

ANOVA demonstrated significant differences among treatments for root length. The MSD method showed differences between the control plants and those treated as biofertilizers. Root length varied between 4.66 and 30.66 cm. No differences in root length were noticed after 30 d in plants inoculated with Azotobacter chroccocum and Trichoderma lignorum, whereas there were no differences among plants sown with Azospirillum brasilense, Azotobacter chroccocum, and Trichoderma lignorum after 45 d. These results can be compared with those reported by Mena-Violante and Olalde-Portugal [21], who found significant increases in root length of tomato plants inoculated with Bacillus subtilis BEB-ISbs (BS13) in relation to control treatments. The increase in the root system length leads to a higher water and nutrient absorption. Moutia et al. [42], achieved an increase of 15% in plant growth (shoot height) and 75% in root dry mass in the sugar cane variety M 1176/77 inoculated with Azospirillum sp.
On day 30, was observed the greatest root length in plants inoculated with Azospirillum brasilense (30.66 cm). This explained by the high production of indoleacetic acid that produce plant growth [43]. Mehnaz and Lazarovits [44], confirmed this theory, when they examined the capacity of Azospirillum lipoferum N7 as a plant-growth promoting agent in corn var. 39D82. Results show that inoculation with biofertilizer microorganisms increases the length of the sugarcane plant root system, indicating a greater growth of absorbent hairs and therefore, greater nutrient catchment [12, 10,11].
ANOVA demonstrated highly significant differences among treatments for the number of roots. The MSD test did not show any differences between Azotobacter chrocooccum and Trichoderma lignorum. On day 15, the control did not present significant differences in relation to the treatment inoculated with Trichoderma lignorum, whereas when Azospirillum brasilense was used, a smaller effect was observed on the studied variable (20 roots) compared to Azotobacter chrocooccum (58 roots), Trichoderma lignorum (50 roots), and the control (51 roots). However, on day 30 and 45 of the experiment, the number of biofertilized sugarcane plant roots increased considerably in relation to the control treatment. Ribaudo et al. [45], evaluated parameters associated with the growth of tomato seeds inoculated with Azospirillum brasilense FT326 where increases were found in the length of the main root hair, root surface, and root and shoot fresh weight. These authors propose ethylene as an intermediary in the signal path stimulating plant growth.
The control produced 68 roots by day 45 while plants inoculated with Azospirillum brasilense, Azotobacter chrocooccum, and Trichoderma lignorum generated 97, 87, and 93 roots, respectively. Treatment with Azospirillum brasilense had the highest number of roots during the last days of the experiment (Figure 5), followed by Trichoderma lignorum. These results can be compared with those reported by Pereyra et al. [46], who found beneficial effects on the root system of wheat plants inoculated with Azospirillim brasilense.

On the other hand, sugar cane has the necessary roots to ensure its maximum growth in optimal soil conditions, which is benefitted by inoculation with Azospirillum strains stimulating root growth. Furthermore, it plays an important role when the plant faces drought conditions [42].
In other studies, inoculation of seaweed with distinct varieties of Azotobacter significantly increased the root blomass mean up to 98.2% in relation to the control treatment [47]. These results agree with those reported by Harman et al. [12], who informed that Trichoderma lignorum strains are able to colonize roots and increase their growth.
Results of root number and length obtained in our study can be compared with those achieved by Baset Mia et al. [48], who showed that inoculation with rhizobacteria (Azospirillum brasilense Sp7 and Bacillus sphaericus UPMB10) and nitrogen supplements stimulated root length and number in banana plants (Musa spp. cv. 'Berangan', type AA) with a 43% root increase in inoculated plants. The authors also compared these results with those obtained when bacterial strains were used without any nitrogen supplement, obtaining significant results. Gamal-Eldin and Elbanna [49], in studies with purple, nonsulfur photosynthetic bacteria (Rhodobacter capsulatus), achieved beneficial effects in terms of high rice plant yield (Oryza sativa).
Number of leaves
Figure 6 shows the variation in the number of leaves for inoculation with Azospirillum brasilense, Azotobacter chrococcum, and Trichoderma lignorum in sugarcane plants var. CC 934418.

The MSD test revealed significant differences among treatments. Inoculation with Trichoderma lignorum showed marked differences with other treatmemnts in the evaluated time periods. This may be due to T. lignorum which is capable of producing compounds such as peptides, proteins, and low-weight molecular compounds that have an antibiotic activity against microorganism pathogens, thus promoting plant growth [50]. Yadav et al. [51], achieved increases in sugar cane nutrient availability and yield in Trichoderma viride applications, trash mulch, and trash burn.
These results can be compared with those obtained by Ravlkumar et al. [47], who found that inoculation with Azotobacter chroococcum stimulated an increase in leaf area in Rhizhopora seedling species. This effect is explained by the capacity of the microorganism to fix atmospheric nitrogen and make it available to the plant. Furthermore, this microorganism in its culture medium secretes hormones such as auxins, gibberelins, and cytokines which stimulate plant growth [52]. Other authors evaluated the effect of fertilizers, such as urea and Azotobacter chroococcum strains on blackberry growth (Morus sdd.) and its leaf quality under saline conditions. They found higher means in the number of primary branches, plant height, and leaf yield in plants supplied with a biological fertilizer. Greater pigment content was also obtained in the last treatment [53]. It is important to mention that foliar application is more effective than soil application because atmospheric nitrogen fixation is sufficient in the phylloplane [54]. Sudhakar et al. [55], also evaluated Azotobacter, Azospirillum, and Beijerinckla as foliar biofertilizers in blackberry plants (Morus spp.) with the Azotobacter treatment showing the best leaf yield. The application of these nitrogen-fixing bacteria improved leaf quality in relation to its protein content. Other authors found positive responses in the leaf area of Punica granatum L plants when Azospirillum brasilense, Azotobacter chroococcum, Glomus fasciculatum, and Glomus mosseae were used as biofertilizers, and where A. brasilense was the microorganism that produced the highest number of leaves [56]. Furthermore, Azotobacter has been reported as producing substances that stimulate seed germination, plant flowering, vigor, yield, and resistance to disease. It has been declared a nitrogen-fixing microorganism which increases soil fertility and productivity of different crops [57]. Gonzalez et al. [58], reported significant changes in fresh weight, adaxial leaf thickness, and root width for in vitro experiments with pineapple inoculated with Azotobacter chroococcum.
CONCLUSIONS
Biofertilizers are a good alternative to using chemical fertilizers in sugar cane crops variety CC 934418. Applying Azospirillum brasilense, Azotobacter chroccocum, and Trichoderma lignorum in sugar cane plants, variety CC 934418, stimulates plant growth. Azospirillum brasilense significantly increased stem diameter, stem length, root number and length. Similarly, beneficial effects of Trichoderma lignorum on leaf growth were observed. This is a new scientific contribution since this species has not been reported as promoting plant growth.
ACKNOWLEGEMENTS
The authors thank the BIOCONTROL Company for financing this research.
REFERENCES
[1] WU, S.C., CAO, Z.H., LI, Z.G., CHEUNG, K.C. and WONG, M.H. Effects of biofertilizer containing N-fixer, P and K solubilizers and AM fungi on maize growth: a greenhouse trial, Geoderma., 125 (1 -2), 2005,p.155-166. [ Links ]
[2] NARULA, N., KUMAR, V., BEHL, R.K., DEUBEL, A., GRANSEE, A. and MERBACH, W. Effect of P-solubilizing Azotobacter chroococcum on N, P, K uptake in P-responsive wheat genotypes grown under greenhouse conditions, J. Plant Nutr. Soil Sc., 163 (4), 2000, p. 393-398. [ Links ]
[3] VESSEY J.K. Plant growth promoting rhizobacteria as biofertilizers, Plant Soil., 255 (3), 2003, p. 571-586. [ Links ]
[4] STURZ, A.V. and NOWAK, J. Endophytic communities of rhizobacteria and the strategies required to create yield enhancing associations with crops, Appl. Soil. Ecol., 15 (2), 2000, p. 183-190. [ Links ]
[5] NELSON, L.M. Plant growth promoting rhizobacteria (PGPR): prospects for new inoculants, Crop Manage., 2004. [ Links ]
[6] BOSTOCK, R.M. Signal crosstalk and induced resistance: straddling the line between cost and Benefit, Annu. Rev. Phytopathol., 43, 2005, p. 545-580. [ Links ]
[7] KOHLER, J., CARAVACA, F., CARRASCO, L. and ROLDAN, A. Contribution of Pseudomonas mendocina and Glomus Intraradices to aggregate stabilization and promotion of biological fertility in rhizosphere soil of lettuce plants under field conditions, Soil Use Manage., 22 (3), 2006, p. 298-304. [ Links ]
[8] CHANDANIE, W.A., KUBOTA, M. and HYAKU-MACHI, M. Interactions between plant growth promoting fungi and arbuscular mycorrhizal fungus Glomus mosseae and induction of systemic resistance to anthracnose disease in cucumber, Plant Soil., 286 (1-2), 2006, p. 209-217. [ Links ]
[9] RAIMAM, M.R, ALBINO, U., CRUZ, M.F., LOVATO, G.M., SPAGO, E., FERRACIN, T.P., LIMA, D.S., GOULART, I., BERNARDI, CM., MIYAUCHI, M., NOGUEIRA, M.A. and ANDRADE, G. Interaction among free-living N-fixing bacteria isolated from Drosera vlllosa var. villosa and AM fungi (Glomus clarum) in rice (Oryza sativa), Appl. Soil Ecol., 35 (1), 2007, p. 25-34. [ Links ]
[10] SPAEPEN, S., VANDERLEYDEN, J. and REMANS, R. Indole-3-acetic acid in microbial and microorganism-plant signaling, Ferns Microbiol. Rev., 31 (4), 2007, p. 425-448. [ Links ]
[11] DARDANELLI, M.S., et al. Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and Nod factor production under salt stress, Soil Biol. Biochem., 40 (11), 2008, p. 2713-2721. [ Links ]
[12] HARMAN, G.E., HOWELL, C.R., VITERBO, A., CHET, I. and LORITO, M. Trichoderma species-opportunistic, avirulent plant symbionts, Nat. Rev. Microbiol., 2 (1), 2004, p. 43-56. [ Links ]
[13] HJELJORD, L.G., STENSVAND, A. and TRONSMO, A. Effect of temperature and nutrient stress on the capacity of commercial Trichoderma products to control Botrytis cinerea and Mucor piriformis in greenhouse strawberries, Biol. Control., 19 (2), 2000, p. 149-160. [ Links ]
[14] LEWIS, J.A. and LUMSDEN, R.D. Biocontrol of damping-off of greenhouse-grown crops caused by Rhizoctonia solani with a formulation of Trichoderma spp, Crop Prot., 20 (1), 2001, p. 49-56. [ Links ]
[15] ROCO, A. and PÉREZ, L.M. In vitro biocontrol activity of Trichoderma harzianum on Alternaria alternata in the presence of growth regulators, Electron. J. Biotechnol., 4 (2), 2001, p. 68-73. [ Links ]
[16] PRASAD, R.D., RANGESHWARAN, R., HEGDE, S.V. and ANUROOP, C.P. Effect of soil and seed application of Trichoderma harzianum on pigeonpea wilt caused by Fusarium udum under field conditions, Crop Prot., 21 (4), 2002, p. 293-297. [ Links ]
[17] CLARKSON, J.R, MEAD, A., PAYNE, T. and WHIPPS, J.M. Effect of environmental factors and Sclerotium cepivorum isolate on sclerotial degradation and biological control of white root by Trichoderma, Plant Pathol., 53 (3), 2004, p. 353-362. [ Links ]
[18] ZAHRAN, H.H. Rhizobia from wild legumes: diversity, taxonomy, ecology, nitrogen fixation and biotechnology, J. Biotechnol., 91 (2-3), 2001, p. 143-153. [ Links ]
[19] RODRÍGUEZ-ECHEVARRÍA, S. and PÉREZ-FERNÁNDEZ, M.A. Potential use of Iberian shrubby legumes and rhizobia inoculation in revegetation projects under acidic soil conditions, Appl. Soil. Ecol., 29 (2), 2005, p. 203-208. [ Links ]
[20] VILLAR-SALVADOR, P., VALLADARES, F., DOMÍNGUEZ-LERENA, S., RUIZ-DÍEZ, B., FERNÁNDEZ-PASCUAL, M., DELGADO, A. and PEÑUELAS, J.L. Functional traits related to seedling performance in the Mediterranean leguminous shrub Retama sphaerocarpa: insights from a provenance, fertilization, and rhizobial inoculation study, Environ. Exp. Bot., 64 (2), 2008, p. 145-154. [ Links ]
[21] MENA-VIOLANTE, H.G. and OLALDE-PORTUGAL, V. Alteration of tomato fruit quality by root inoculation with plant growth-promoting rhizobacteria (PGPR): Bacillus subtilis BEB-13bs, Sci. Hortic-Amsterdam., 113 (1), 2007, p. 103-106. [ Links ]
[22] ESITKEN, A., KARLIDAG, H., ERCISLI, S., TURAN, M. and SAHIN, F. The effect of spraying a growth promoting bacterium on the yield, growth and nutrient element composition of leaves of apricot (Prunus armeniaca L. cv. Hacihaliloglu), Aust. J. Agr. Res., 54 (4), 2003, p. 377-380. [ Links ]
[23] DEY R., PAL, K.K., BHATT, D.M. and CHAUHAN, S.M. Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria, Microbiol. Res., 159 (4), 2004, p. 371-394. [ Links ]
[24] PUENTE, M.E., LI, C.Y. and BASHAN, Y. Microbial populations and activities in the rhizoplane of rock-weathering desert plants. II. Growth promotion of cactus seedlings, Plant Biology., 6 (5), 2004, p. 643-650. [ Links ]
[25] KIZILKAYA, R. Yield response and nitrogen concentrations of spring wheat (Triticum aestivum) inoculated with Azotobacter chroococcum strains, Ecol. Eng., 33 (2), 2008, p. 150-156. [ Links ]
[26] URQUIAGA, S., CRUZ, K.H.S. and BODDEY R.M. Contribution of nitrogen fixation to sugarcane: nitrogen-15 and nitrogen-balance estimates, Soil Sci. Soc.Am. J., 56 (1), 1992, p. 105-114. [ Links ]
[27] GRIVET, L., ARRUDA, P. Sugarcane genomics: Depicting the complex genome of an important tropical crop, Curr. Opin. Plant Biol., 5 (2), 2002, p. 122-127. [ Links ]
[28] REIS, V., LEE, S. and KENNEDY, C. Associative and Endophytic Nitrogen-Fixing Bacteria and Cyanobacterial Associations. Biological nitrogeno fixation in sugarcane. Emerich C, Newton W.E. (ed.) Dordrecht, The Netherlands: Springer, 2007, p. 213-232. [ Links ]
[29] MIRZA, M.S., AHMAD, W., LATIF, F., HAURAT, J., BALLY, R., NORMAND, P. and MALIK, K.A. Isolation, partial characterization, and the effect of plant growth-promoting bacteria (PGPB) on micro-propagated sugarcane in vitro, Plant Soil., 237 (1), 2001, p. 47-54. [ Links ]
[30] SUMAN, A., SHASANY A.K., SINGH, M., SHAHI, H.N., GAUR, A. and KHANUJA, S.P.S. Molecular assessment of diversity among endophytic diazotrophs isolated from subtropical Indian sugarcane, World J. Microb. Biot., 17 (1), 2001, p. 39-45. [ Links ]
[31] STAMFORD, N.R, LIMA, R.A., LIRA J.R., M.A. and SANTOS, C.R.S. Effectiveness of phosphate and potash rocks with Acidithiobacillus on sugarcane yield and their effects on soil chemical attributes, World J. Microb. Biot., 24 (10), 2008, p. 2061-2066. [ Links ]
[32] RAMAMURTHY, V., NAIDU, L.G.K., RAMESH KUMAR, S.C., SRINIVAS, S. and HEGDE, R. Soil-based fertilizer recommendations for precision farming, Curr. Sci, 97 (5), 2009, p. 641-647. [ Links ]
[33] SRINIVASRAO, C.H. and VITTAL, K.R.R. Emerging nutrient deficiencies in different soil types under rainfed production systems of India, Indian J. Fert., 3, 2007, p. 37-46. [ Links ]
[34] SINGH, S. and DATTA, P. Outdoor evaluation of herbicide resistant strains of Anabaena variabilis as biofertilizer for rice plants, Plant Soil., 296 (1 -2), 2007, p. 95-102,. [ Links ]
[35] DUNNE, C, MOËNNE-LOCCOZ, Y, MCCARTHY J., HIGGINS, P., POWELL, J., DOWLING, D.N. and O'GARA, F. Combining proteolytic and phloroglucinol-producing bacteria for improved biocontrol of Pythium- mediated damplng-off of sugar beet, Plant Pathol., 47 (3), 1998. p. 299-307. [ Links ]
[36] SHOEBITZ, M., RIBAUDO, CM., PARDO, M.A., CANTORE, M.L., CIAMPI, L. and CURA, J.A. Plant growth promoting properties of a strain of Enterobacter ludwigii isolated from Lollum perenne rhizosphere, Soil Biol Biochem, 41 (9), 2009, p. 1768-1774. [ Links ]
[37] SENTHILKUMAR, M., GOVINDASAMY V. and ANNAPURNA, K. Role of antibiosis in suppression of charcoal rot disease by soybean endophyte Paenibacillus sp. HKA-15, Curr Microbiol., 55 (1), 2007, p. 25-29. [ Links ]
[38] RUSSO, A., VETTORI, L., FELICI, C., FIASCHI, G., MORINI, S. and TOFFANIN, A. Enhanced micropropagation response and biocontrol effect of Azospirillum brasilense Sp245 on Prunus cerasifera L. clone Mr.S 2/5 plants, J. Biotechnol., 134 (3-4), 2008, p. 312-319. [ Links ]
[39] DONOSO, E., LOBOS, G.A. and ROJAS, N. Efecto de Trichoderma harzianum y compost sobre el crecimiento de plántulas de Pinus radiata en vivero, Bosque (Valdivia)., 29 (1), 2008, p. 52-57. [ Links ]
[40] LORITO, M., WOO, S.L. and SCALA, F. Le biotecnologie utili alia difusa sostenibile delle piante: i fungí, Agroindustria., 3, 2004, p. 181-195. [ Links ]
[41] SHUKLA, SK., YADAV, RL, SUMAN, A. and SINGH, PN. Improving rhizospheric environment and sugarcane ratoon yield through bloagents amended farm yard manures in udic Ustochrept soil, Soil Till. Res., 99 (2), 2008, p. 158-168. [ Links ]
[42] MOUTIA, J.F.Y, SAUMTALLY S., SPAEPEN, S. and VANDERLEYDEN, J. Plant growth promotion by Azospirillum sp. in sugarcane is influenced by genotype and drought stress, Plant Soil., 337, 2010, p. 233-242. [ Links ]
[43] MOLLA, A.H., SHAMSUDDIN, Z.H. and SAUD, H.M. Mechanism of root growth and promotion of nodulation in vegetable soybean by Azospirillum brasilense, Commun. Soil Sci Plant., 32 (13-14), 2001, p. 2177-2187. [ Links ]
[44] MEHNAZ, S. and LAZAROVITS, G. Innoculation effects of Pseudomonas putida, Gluconacetobacter azotocaptans, and Azospirillum lipoferum on corn plant growth under greenhouse conditions, Microbial Ecol., 51 (3), 2006, p. 326-335. [ Links ]
[45] RIBAUDO, CM., KRUMPHOLZ, E.M., CASSÁN, F.D., BOTTINI, R., CANTORE, M.L and CURÁ, J.A. Azospirillum sp. Promotes Root Hair Development in Tomato Plants through a Mechanism that Involves Ethylene, J. Plant Growth Regul., 25,2006, p. 175-185. [ Links ]
[46] PEREYRA, M.A., BALLESTEROS, F.M., CREUS, CM., SUELDO, R.J. and BARASSI, C.A. Seedlings growth promotion by AzospIrillum brasIlense under normal and drought conditions remains unaltered in Tebuconazole-treated wheat seeds, Eur. J. Soil Biol., 45 (1), 2009, p. 20-27. [ Links ]
[47] RAVI KUMAR, S., KATHIRESAN, K., THADEDUS MARIA IGNATIAMMAL, S., BABU SELVAM, M. and SHANTHY, S. Nitrogen-fixing azotobacters from mangrove habitat and their utility as marine biofertilizers., J. Exp. Mar. Biol. Ecol., 312 (1), 2004, p. 5-17. [ Links ]
[48] BASET MIA, M.A., SHAMSUDDIN, Z.H., WAHAB, Z. and MARZIAH, M. Rhizobacteria as bioenhancer and biofertilizer for growth and yield of banana (Musa spp. cv. 'Berangan'), Sci Hortic-Amsterdam., 126 (2), 2010, p. 80-87. [ Links ]
[49] GAMAL-ELDIN, H. and ELBANNA, K. Field Evidence for the Potential of Rhodobacter capsulatus as Biofertilizerfor Flooded Rice, Curr. Microbiol., 62 (2), 2010, p. 391-395. [ Links ]
[50] REINO, J.L., GUERRERO, R.F., HERNÁNDEZ-GALÁN, R. and COLLADO, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma, Phytochemistry Reviews., 7 (1), 2007, p. 89-123. [ Links ]
[51] YADAV, R.L., SHUKLA, S.K., SUMAN, A. and SINGH, P.N. Trichoderma inoculation and trash management effects on soil microbial biomass, soil respiration, nutrient uptake and yield of ratoon sugarcane under subtropical conditions, Biol. Fert. Soils., 45 (5), 2009, p. 461-468. [ Links ]
[52] MEHBOOB, I., NAVEED, M. and ZAHIR, Z.A. Rhizobial Association with Non-Legumes: Mechanisms and Applications, Cr. Rev. Plant Sci, 28 (6), 2009, p.432-456. [ Links ]
[53] VIJAYAN, K., CHAKRABORTI, S.R and GHOSH, P.D. Foliar application of Azatobactor chroococcum increases leaf yield under saline conditions in mulberry (Morus spp.), Sci. Hortic-Amsterdam., 113 (3), 2007, p. 307-311. [ Links ]
[54] SAXENA, A.K. and TILAK, K.V.B.R. Interaction among beneficial soil microorganisms, Indian J. Microbiol., 34,1994, p. 91-106. [ Links ]
[55] SUDHAKAR, P., CHATTOPADHYAY G.N., GANGWAR, S.K. and GHOSH, J.K. Effect of foliar application of Azotobacter, Azospirillum and Beijerinckia on leaf yield and quality of mulberry (Morus alba), J Agr Sci., 134, 2000 (2), p. 227-234. [ Links ]
[56] ASERI, G.K., JAIN, N., PANWAR, J., RAO, A.V. and MEGHWAL, P.R. Biofertilizers improve plant growth, fruit yield, nutrition, metabolism and rhizosphere enzyme activities of Pomegranate (Púnica granatum L.) in Indian Thar Desert, Sci Hortic-Amsterdam.,117 (2), 2008, p. 130-135. [ Links ]
[57] SASHIDHAR, B. and PODILE, A.R. Mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway glucose dehydrogenase, J Appl Microbiol., 109 (1), 2010, p. 1-12. [ Links ]
[58] GONZÁLEZ, R., LAUDAT, T., ARZOLA, M., MÉNDEZ, R., MARRERO, P., PULIDO, L.E., DIBUT, B. and LORENZO, J.C. Effect of Azotobacter chroococcum on in vitro pineapple plants growth during acclimatization, In Vitro Cell. Dev. B., 47 (3), 2010, p. 387-390. [ Links ]














