Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Earth Sciences Research Journal
Print version ISSN 1794-6190
Earth Sci. Res. J. vol.13 no.2 Bogotá July/Dec. 2009
CHARACTERIZATION OF VERMICULITE BY XRD AND SPECTROSCOPIC TECHNIQUES
A. Campos, S. Moreno, R. Molina1
1 Estado sólido y catálisis ambiental, Departamento de Química, Facultad de Ciencias, Universidad Nacional de Colombia. AK 30 No. 45-03, Bogotá, Colombia; Fax: 57-1-3165220
E-mail Autor:ramolinag@unal.edu.co
Manuscript received: 24/03/2009 Accepted for publication: 26/05/2009
ABSTRACT
A natural mineral from Santa Marta-Colombia used as the starting material in the synthesis of pillared clays has been characterized by several techniques, including X-ray diffraction, X-ray fluorescence, electronic paramagnetic resonance, aluminum nuclear magnetic resonance and scanning electron microscopy. The information revealed that the mineral corresponds to trioctahedral vermiculite. The identification of this mineral is valuable in the control of reduction charge and pillaring processes on these materials to obtain more complex solids like the ones required to specific catalytic applications.
Key words: Vermiculite, structural formula, layer charge.
RESUMEN
Un mineral natural de la región de Santa Marta en Colombia, el cual usado como el material de partida en la síntesis de arcillas pilarizadas, ha sido caracterizado por diversas técnicas tales como difracción de rayos-X, fluoresencia de rayos-X, resonancia electrónica paramagnética, resonancia magnética nuclear de aluminio y microscopía electrónica de barrido. La información en conjunto indica que el mineral corresponde a vermiculita trioctaédrica. La identificación de este mineral es muy importante en la comprensión y el control de los procesos de reducción de carga y de pilarización de estos materiales para la obtención de sólidos más complejos, como los requeridos en aplicaciones catalíticas específicas.
Palabras clave: Vermiculita, fórmula estructura, capa de carga.
1. Introduction
Vermiculite is a clay mineral usually of secondary origin due to the alteration of mica, pyroxene, chlorite or similar minerals (Brown 1961).
The substitutions of Si by Al in tetrahedral sheet predominate in vermiculite. In this way, the negative charge generated on the tetrahedral sheets limits the expansion properties of the clay and this factor determines the high layer staking of its structure (Mac Ewan and Wilson 1980; Tunega et al. 2003).
From the catalytic point of view, the vermiculite is a very attractive material due to the thermal resistance (Suvorov and Skurikhin 2003) and the number oftetrahedral substitutions, which ensure the presence of a larger number of Br0nsted-type acid sites (del Rey-Pérez-Caballero and Poncelet et al. 2000); these substitutions are less numerous in other hydrous 2: 1 minerals such as smectites. On the whole, these properties offer considerable interest in the production of pillared clays, which are distinguishable from an intercalated layered solid due to their micro and/or mesoporosity and high thermal stability with preservation of the layer stacking (Moreno et al. 1997, Schoonheydt et al. 1999; Stefanis etal. 2006).
The development of pillared clays from vermiculite during the past few years has offered particular interest especially as a heterogeneous catalyst in acid-catalyzed reactions (Campos et al. 2005; Campos et al. 2007; Campos and Gagea et al. 2008; Campos and Moreno et al. 2008; Cristiano et al. 2005; del Rey-Pérez-Caballero and Poncelet et al. 2000; del Rey-Pérez-Caballero and Sánchez et al. 2000, Hernández et al. 2007). However, as direct intercalation of vermiculite is impossible due to a very high potential of stabilization of interlayer cations (Suvorov and Skurikhin2003) it has been necessary to develop alternative methodologies to achieve pillared vermiculite (Cristiano et al. 2005; F. del Rey-Pérez-Caballero 2000).
One of these methodologies has been the hydrothermal treatmentprior to a conventional process ofintercalation, in order to reduce negative charge and make possible the subsequent cationic exchange (Cristiano et al. 2005). The catalytic profile of Al and Al-Zr modified vermiculites by means of this methodology has confirmed the potential expected for this acidic acid solid, since they are highly active with respect to other clays in alkane hydroisomerization (Campos 2005; Campos et al. 2007; Campos and Gagea et al. 2008; Campos and Moreno et al. 2008; Cristiano et al. 2005; del Rey-Pérez-Caballero and Poncelet 2000; del Rey-Pérez-Caballero and Sánchez et al. 2000; Hernández et al. 2007).
In order to produce practical information to control the modification processes described previously, to achieve a clay mineral with potential catalytic and adsorptive properties, this paper focuses on the characterization of natural vermiculite issued from a Colombian deposit. X-ray diffraction (XRD) and several spectroscopic techniques such as electronic paramagnetic resonance (EPR) and nuclear magnetic resonance (NMR)wereused.
2. Experimental
2.1 Materials
The vermiculite selected for this work was labeled V, and it corresponds to a commercial mineral, which comes from a natural deposit in the Santa Marta region in Colombia. Its characterization was carried out with the fraction of a particle size smaller than 150 μm, separated from the commercial form by sieve, without purification or fractioning treatment.
2.2 Characterization methods
The cationic exchange capacity was determined on the clay previously interchanged with an ammonium acetate solution, by means of the micro-Kjeldahl method (Chapman 1965).
The X-ray diffraction study obtained from flakes, which had been gently pressed onto the plates by using a Philips PW 1820 diffractometer (Kα radiation of Cu, λ=1.54056 Å, 40 mA, 40 kV) in 2θ geometry and Bragg-Brentano configuration, a step size of 0.05 and a step time of 2 s. Diffraction patterns were taken at room temperature, 20 °C and 65% of humidity on the average.
The identification of the mineral was assessed according to the position of basal reflection 001 in three patterns: i) natural sample, ii) after solvation in the presence of ethylene glycol for 24h and iii) after heating at 500 °C for 2 h. In addition, Mg2+ and K+ saturations were performed in the mineral to achieve a better characterization (Thorez 1976).
Adsorption-desorption isotherms of nitrogen at the temperature of liquid nitrogen were analyzed using a Micromeritics Tristar 3000 instrument on samples previously degassed at 200 °C for 6 h.
Scanning electron microscopy images were recorded in a Philips Scanning Electron Microscope XL30 FEG.
The gravimetric and differential thermal-analysis were performed at a heating speed of 10 °C min-1 in air atmosphere using a Thermal Analyzer TG and DSC Rheometric instruments.
The chemical analysis was performed by X-ray fluorescence in an XRF 2400 instrument. In addition, the Fe2+ analysis was carried out through the analytic method described in Wilson M. (Wilson 1995).
Electron paramagnetic resonance spectra in the X band (9.8 GHz) was carried out in a Brucker ESP 3220 spectrometer adopting a modulation frequency of 100 kHz whose width was fixed at 0.4 mT for wide lines and reduced to 0.05 mT for acute bands, at a temperature of 100 K.
The nuclear magnetic resonance of 27Al was performed in a Brucker Advance 400 spectrometer; the sample was placed in 2.5mm rotors, while 1200 scannings were accumulated with a time of 100ms of recycle. Rotor speed was 5000 Hz.
XRF, EPR and NMR measurements were done additionally in the mineral washed with nitric acid 0.01 M (10 ml g-1 clay) by 1h at 30 °C.
3. Results and discussion
3.1 X-ray fluorescence (XRF) and cation exchange capacity (CEC)
Table 1 records the chemical composition of the mineral obtained by X-ray fluorescence. The structural formula of the Colombian vermiculite estimated from its chemical composition and following the methodology described in Wilson M. (Wilson 1995) is:
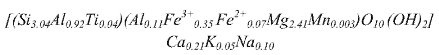
On the other hand, the cationic exchange carried out with ammonium-vermiculite is 1.10 meq 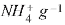 .
.
3.2 X-ray diffraction analysis
Table 2 shows the comparison between the reflections in XRD for Mg-vermiculite (Brown 1961), those corresponding to a diffraction pattern calculated using DIFK software (Wiewióra et al. 2003) and the reflections observed in the pattern for natural vermiculite V.
This parallel makes it possible to observe an analogue pattern between the clays; thus the clay mineral under study corresponds to vermiculite (Wiewióra et al. 2003). Likewise, a high layer stacking is highlighted, which is verified by the presence of reflections in series 02l and 11 l (Wiewióra et al. 2003).
Reflection 060 located in 0.154 nm indicates that the vermiculite structure is of the trioctahedral type (Dixon and Weed 1989). In general, vermiculite can be dioctahedral or trioctahedral, but trioctahedral type is common in soils with a similar morphology to that of mica (Moore and Reynolds 1997), as observed in vermiculite (Figure 3).
On the other hand, 6th,8th, and 10th order reflections located at 0.480, 0.360 and 0.288 nm respectively show a growing intensities serie typical for vermiculites (Wiewióra et al. 2003). Interstratified material is absent; otherwise the sequence should be abnormal (Wiewióra et al. 2003).
Trioctahedral vermiculites usually formed bythe weathering of biotite (Gordeeva et al. 2002) may be found in the company of this mineral and also talc is possible. In the case of clay V we can suggest that talc is found as a non significant impurity, indicated by its characteristic diffractions in 0.93 nm, 0.48 nm and 0.31 nm (Fagel et al. 2001) (Figure 1). In parallel, starting from the positions of reflections 001 and 004 it can be estimated that the vermiculite is found in a proportion larger than 90 % (Thorez 1976).
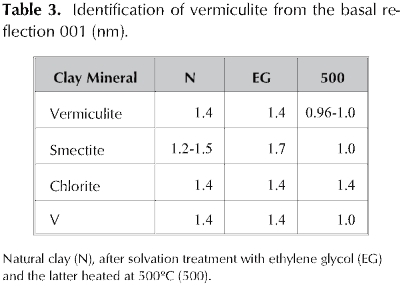
In order to verify the purity of the mineral, Table 3 records the follow up on reflection 00l of raw natural clay powder, when the sample is solvated in the presence of ethylene glycol and, when the latter is calcined at 500 °C. This table shows that the V material exhibits the sequence typical of vermiculites (Thorez 1976; Dixon and Weed 1989).
However different authors (Thorez 1976, Dixon and Weed 1989, Malla and Douglas 1987) suggest complementary analysis XRD in Mg2+ and K+-vermiculite to distinguish between soil vermiculites and OH-interlayer vermiculites. As well they suggest a relation between the d001 signal and the layer charge.
After saturation with magnesium followed by glycerol the basal reflection should be located in 1.4 nm compared with 1.8 nm if the clay 2:1 were a smectite (Thorez 1976; Fagel et al. 2001). In this sense, the basal reflection of V located at 1.4 nm was not modified. The impossibility for expansion in Mg-V is then a consequence of the strong retention of magnesium between layers with a high negative density like vermiculites (Slade et al. 1976, Tunega et al. 2003) (Table 4).
Malla and Douglas (Malla and Douglas 1987) established that the saturation with this potassium originates interlayer space reduction in the region between 1.12 -1.20 nm if the charge is lower than 0.57 and, between 1.00 and 1.06 nm when it is at least 0.6.
In this sense, when clay V was saturated in the presence of K+ the basal spacing of 1.4 nm was reduced to 1.0 nm after dried at room temperature and calcinations ofthe sample at 500 °C (Table 4). The reduction of interlayer space after potassium saturation, qualitatively indicate that the layer charge must be higher than 0.57 (Malla and Douglas 1987).
In addition, the previous characteristic is a tool for the clear distinction between vermiculite and chlorite, due to the fact that the latter keeps the 001 reflection at 1.4 nm when K-chlorite, while the basal spacing of K-vermiculite is reducedto 1.0 nm (Dixon and Weed 1989). For this reason, the absence of chlorite is verified as a possible impurity in the mineral.
3.3 Thermal analysis
The thermal-gravimetric (TG) and differential (DTA) analysis of vermiculite is presented in the Figure 2 where a total weight loss of 21.1 % is distributed in several regions. In the first one, the dehydration of the clay takes place in two stages: i) between 20 and 110 °C which corresponds to the loss of water physically absorbed on the surface and ii) from 110 °C to 500 °C, with a weight loss of 14.3 % attributed to the exit of water molecules, which are in contact with the cations in the interlayer region (Pérez et al. 2003).
The region between 500 and 850 °C records a loss of 6.8 % in weight with a linear tendency with respect to temperature, which is attributed to dehydroxilation (Pérez-Rodríguez et al. 2004). In fact, the unusual endothermic peak at 800 °C is associated with the thermal stability on vermiculite which is characteristic of this mineral as well this signal could be assigned to the formation of a new enstatite crystalline phase, which has been reported previously (Pérez et al. 2003).
3.4 Texture and morphology
The selected fraction for vermiculite V corresponds to the mineral observed in Figure 3, where large vermiculite layer crystals are detected, which display soft surfaces, with small protuberances. Polygonal sheets with flaked borders are observed in vermiculite formed by the alteration of parental materials (Kishk and Barshad 1969).
The specific surface area (7.41 m2 g-1) corresponding to external surface area without any microporosity. The low surface area is related to the particle but as well with the surface charge, since micas with similar particle size and charge higher than vermiculites have less surface area (del Rey-Pérez-Caballero and Sánchez et al. 2000).
The strong hydrogen bonds between the tetrahedral sheet and the water of interlayer cations in vermiculite can keep the interlayer zone obstructed (Tunega D. et al. 2003), which could reduce superficial nitrogen adsorption considerably.
By other hand, the relation observed between the interchange cations and the penetration in the interlayer space has been indicated by different authors (Pérez et al. 2003). In fact, when monovalent cations in the interlayer region are replaced by those of divalent type, a significant reduction in the superficial area for this mineral clay is observed (Pérez et al. 2003).
3.5 Electronic paramagnetic resonance (EPR) and nuclear magnetic resonance of aluminum (27Al-NMR)
Natural vermiculite can be accompanied by iron oxides and hydroxides on its surface (iron that is not substituting aluminum in the octahedral sheet), which will cause an overestimation of iron in vermiculite if it is evaluated by chemical analysis. In this way, EPR could be used to determine the presence of structural Fe3+ in vermiculite. Additionally, to evaluate the structural environment of paramagnetic ions found in the clay.
As can be observed in Figure 4, the EPR of V is dominated by signals associated with Fe3+ g = 4.3 and 2.1. In addition, a sextet of lines in g = 2 can be appreciated, which is typical of Mn2+ species with distorted octahedral symmetry (Tus¡ar et al. 2005).
The signal at g = 4.3 is related with Fe3+ located at an orthorhombic environment within the clay structure (Bensimon et al. 2000). In general, smectites exhibit signals for Fe3+ between g = 4.3 and g = 2.0, which can be attributed to the combination of two different octahedral and two tetrahedral environments. Their formation depends on the possible organizations of the OH groups in the FeO4(OH)2 unit (Wilson1995).
Mc Bride et al. (Mc Bride et al. 1975) suggest that the difference between the two octahedral sites occupied by Fe3+ ions depends on the nature of the adjacent octahedral cation (divalent or trivalent). According to Timofeeva et al. (Timofeeva et al. 2005), the iron found in the form of aggregates in the surface is related with signals in the region comprised between g = 2.3-2.6. Alternatively, different contributions to the wide signal located around g = 2.1 are related with FexOy or FeOOH species (Chung et al. 2004).
In the case of the smectite minerals the signal at g = 4.3 is assigned to the presence of isolated Fe3+ in tetrahedral or octahedral coordination, which corresponds to the iron located in the interior of the clay sheets (iron substituting aluminum in the octahedral layers). On the other hand, the signal at g = 2.0 is associated to the presence of clusters of iron (iron extra-red species) (Carriazo et al. 2005).
As observed in Figure 4a, several signals point to the presence of iron extra-red species on the surface of the clay V. For this reason, the clay was submitted to a soft acid wash to remove impurities from its surface. In Figure 4b, the absence of signals between g = 2.3-2.6 indicates that these species had been removed.
Likewise, signals of Mn2+ ions located in the region at g = 2, and g = 1.9 (Figure 4a), and the multiple signal recorded is interpreted in terms of the interaction between structural ions Mn2+ and Fe3+ and other environments (Kessissoglou 1999). After acid wash Mn2+ signals remain invariable which suggest structural positions for this cation.
Respect to aluminum, in order to evaluate the structural environment as well as the AL(IV)/AL(VI) ratio to compare with the ratio estimated in the structural formula, 27Al MAS-NMR spectra was made. The spectrum is shown in Figure 5, in which an intense signal at 60 ppm is observed (with two sidebands at -60 and 190 ppm). This signal is associated with Al(IV) substituting the silica in the tetrahedral sheets (Klinowski 1999). A second signal corresponding to Al(IV) at 0 ppm (Klinowski 1999) was observed. The intensity ratio Al(IV)/Al(VI) was 10.4.
3.6 Correlation of Results
Weaver (Weaver 1958) suggested that vermiculite derived from mica weathering has a high laminar charge and its 001 reflection decrease to 1.0 nm after potassium saturation, as happens in clay V (Table 4). In contrast, vermiculites generated by the weathering of materials like amphiboles or volcanic material exhibit a limited layer contraction with the same treatment.
In fact, the laminar charge is a fundamental element in identification of 2:1 silicates. In fact, the AIPEA (International Association for the Study of Clays) established that vermiculite is a phylosilicate 2:1 with a charge of 0.6 to 0.9 per unit cell (Guggenheim et al. 2006). In this sense, to determine the layer charge of V, it was performed with the structural formula, which was determined through XRF elemental analysis. In it formula can be deduced that the tetrahedral sheet originate an excess negative charge of -0.9 e/ O10(OH)2, which is compensated by Ca2+,Na+ and K+ cations. As regards to the octahedral sheet, formed specially by Fe3+ and Mg2+ it carries a positive charge of 0.35, resulting in a total negative charge of 0.6 e- / O10(OH)2.
In order to confirm the approach to the elemental organization and to evaluate the environment of the elements found in the structure, EPR and NMR provide very valuable information. Thus, by means of EPR spectroscopy, iron species on the natural clay surface were detected, which can be easily removed through a soft acid wash (Figure 4b). In consequence, the chemical analysis by XRF to evaluate the structural formula was done in the mineral washed with soft acid because unwashed sample could overestimate the iron content.
Regarding the NMR the Al (IV)/Al (VI) ratio (10.4) compared with the ratio estimated with the structural formula (8.9) is a satisfactory approach of vermiculite structure take into account that paramagnetic species such as iron can modify the tetrahedral/octahedral ratio (Gates et al. 1996).
The total negative charge (Malla and Douglas 1987; Guggenheim et al. 2006) observed in the structural formula and the slight difference with the CEC as well as the XRD signals and the follow up of the basal reflection 001 after submitting the sample to different treatments (Thorez 1976; Wilson 1995; Moore and Reynolds 1997) indicate that mineral V is trioctahedral vermiculite.
The knowledge derived from the present work is very useful in further understanding, controlling and predicting the modifications that can be performed on this attractive natural mineral in order to obtain materials with technological impact such as catalyst and adsorbents (Campos et al. 2005; Cristiano et al. 2005; Campos et al. 2007; Campos and Gagea et al. 2008; Campos and Moreno et al. 2008, Hernández et al. 2007).
5. Conclusion
Mineral clay from a deposit in the region ofSanta Marta, Colombia has been characterized through different techniques. The correlation of results indicates that the mineral corresponds to trioctaheral vermiculite as result of the mica-bio-tite weathering process. The layer stacking, its high thermal stability, low superficial area and the magnitude ofits negative charge are characteristic of vermiculite. The details about the structure of this mineral are highly useful in the latter pillaring process of these mineral to enhance their catalytic applications.
Acknowledgements
This research has been supported by Projects Code HERMES 9887 VRI-DIB-Universidad Nacional de Colombia. The authors wish to thank Professor Pierre Jacobs, director of the Centrum voor Oppervlaktechemie en Katalyse at Katholieke University of Leuven for carrying out the NMR and EPR analysis.
References
Bensimon Y., Deroide B., Dijoux F., Martineau M. (2000). Nature and thermal stability of paramagnetic defects in natural clay: a study by electron spinresonance. Journal of Physics and Chemistry of Solids 61, 1623-1632. [ Links ]
Brown B. (1961) TheX-Ray identification and crystal structures ofclay minerals. Mineralogical Society; London. [ Links ]
Campos A., Moreno S., Molina R. (2005). Acidez e hidroisomerización de heptano en una vermiculita colombiana modificada con aluminio. Revista Colombiana de Química. 34, 79-92. [ Links ]
Campos A., Gagea B., Moreno S., Jacobs P., Molina R. (2007). Hydroisomerization of decane on Pt/Al,Ce-pil-lared vermiculites. Studies in Surface Science and Catalysis. 170, 1405-1409. [ Links ]
Campos A., Gagea B., Moreno S., Jacobs P., Molina R. (2008). Decane hydroconversion with Al-Zr, Al-Hf, Al-Ce-pillared vermiculites. Applied Catalysis A. 345, 112-118. [ Links ]
Campos A., Moreno S., Molina R. (2008). Relationship between hydrothermal treatment parameters as a strategy to reduce layer charge in vermiculite, and its catalytic behavior. Catalysis Today. 133-135, 351-356. [ Links ]
Carriazo J., Guélou E., Barrault J., Tatibouêt J., Molina R., Moreno S., 2005. Synthesis of pillared clays containing Al, Al-Fe or Al-Ce-Fe from a bentonite: Characterization and catalytic activity. Catalysis Today 107-108,126-132. [ Links ]
Chapman H. (1965). Cation exchange capacity. In: Black C, Evans D, White J, Ensminger L, Clark E, editors. Methods of Soil Analysis (Agronomy 9), American Society of Agronomy. [ Links ]
Chung H., Sang-Won C., Yong-Sik O., Jinho J. (2004). EPR characterization of the catalytic activity of clays for PCE removal by gamma-radiation induced by acid and thermal treatments. Chemosphere 57, 1383-1387. [ Links ]
Cristiano D., Campos A., Molina R. (2005). Charge reduction in a vermiculite by acid and hydrothermal methods: A comparative study. Journal ofPhysical Chemistry B 109, 19026-19033. [ Links ]
del Rey-Pérez-Caballero F., Poncelet G. (2000). Microporous 18 Ã Al-pillared vermiculites: preparation and characterization. Microporous and Mesoporous Materials. 37, 313-327. [ Links ]
del Rey-Pérez-Caballero F., Sánchez M., Poncelet G. (2000). Hydroisomerization of octane on Pt/Al-pillared vermiculite, and comparison with zeolites. Studies in Surface Science and Catalysis. 130, 2417-2422. [ Links ]
Dixon J., Weed S. (1989). Minerals in soil environments. Second edition. United States. [ Links ]
Fagel N., Robert C., Preda M., Thorez J. (2001). Smectite composition as a tracer of deep circulation: the case of the Northern North Atlantic. Marine Geology. 172, 309-330. [ Links ]
Gates W., Stucki J., Kirkpatrick R. (1996). Structural properties of reduced Upton montmorillonite. Physics and Chemistry ofMinerals 2, 535-541. [ Links ]
Gordeeva L., Moroz E., RudinaN., Aristov Y. (2002). Formation of porous vermiculite structure in the course of swelling. Russian Journal ofApplied Chemistry. 75, 357-361. [ Links ]
Guggenheim S., Adams J., Bain B., Bergaya F., Brigatti M., Drits V., Formoso M., Galán E., Kogure T., Stanjek H. (2006). Summary of recommendations of nomenclature committees relevant to clay mineralogy: report of the association international pour l'etude des argiles (AIPEA) nomenclature committee for 2006. Clays and ClayMinerals. 54, 761-772. [ Links ]
Hernández W., Centeno M., Odriozola J., Moreno S., Molina R. (2007). Acidity characterization ofa titanium and sulfate modified vermiculite. Materials Reserach Bulletin. 43, 1630-1640. [ Links ]
Kessissoglou D. (1999). Homo- and mixed-valence EPR -active trinuclear manganese complexes. Coordination Chemistry Review. 185, 837-858. [ Links ]
Kishk F., Barshad I. (1969). The morphology ofvermiculite clay particles as affected by their genesis. American Mineralogist. 54, 849-857. [ Links ]
Klinowski J. (1999). Solid-state NMR studies of molecular sieve catalysts. Chemical Reviews. 91, 1459-1479. [ Links ]
Laird D. (1999). Layer Charge Influences on the Hydration ofExpandable 2:1 Phyllosilicates. Clays and ClayMinerals. 47, 630-636. [ Links ]
Malla P., Douglas L. (1987). Layer charge properties of smectites and vermiculites: tetrahedral vs. Octahedral. Soil Science Society of America Journal. 51, 1362-1366. [ Links ]
MacEwan, D. and Wilson, M. (1980). Interlayerand intercalation complexes of clay minerals. Pp. 197 248 in: Crystal Structures ofClay Minerals and Their X-ray Identification (G.W. Brindley and G. Brown, editors). Mineralogical Society, London. [ Links ]
Mc Bride M., Pinnavaia T., Mortland M. (1975). Exchange Ion Positions inSmectite: Effects on Electron Spin Resonance of Structural Iron. Clays and Clay Minerals. 23,162-164. [ Links ]
Moreno S., Gutiérrez E., Alvarez A., Papayannakos N., Poncelet G. (1997). Al-pillared clays: from lab syntheses to pilot scale production characterization and catalytic properties. Applied Catalysis A 165, 103-114. [ Links ]
Moore D., Reynolds J. (1997) . X-ray diffraction and the identification andanalysis ofclayminerals. Secondedition. Oxford university press. [ Links ]
Pérez L., Balek V., Potayo J., Pérez J., Subrt J., Bountsewa I., Beckman I., Málek Z. (2003). Study of natural and ion exchanged vermiculite by emanation thermal analysis, TG, DTA and XRD. Journal of Thermal Analysis and Calorimetry. 71, 715-726. [ Links ]
Pérez-Rodriguez J., Potayo J., Jiménez de Haro M., Pérez-Maqueda L., Lerf A. (2004). Thermal decomposition of NH+ -vermiculite from Santa Olalla (Huelva, Spain) and its relation to the metal ion distribution in the octahedral sheet Physics and ChemistryofMinerals 31, 415-420. [ Links ]
SchoonheydtR., PinnavaiaT., LagalyG., GangasN. (1999). Pillared clays and pillared layered solids. Pure Applied Chemistry. 71: 2367-2371. [ Links ]
Slade P. (1976). Telleria M., Radoslovic W. The estructures of ornithine-vermiculite and 6-aminohexanoic acid-ver-miculite. Clays and Clay Minerals 24, 134-141. [ Links ]
Suvorov D., Skurikhin V. (2003). Vermiculite: A promising material for high-temperature heat insulators. Refractories and Industrial Ceramics. 44, 186-193. [ Links ]
Stefanis A., Tomlinson A. (2006). Towards designing pillared clays for catalysis. Catalysis Today 114,126-141. [ Links ]
Thorez J. (1976). Practical identification ofclay minerals. G. Lelotte (Ed). Dison, Belgique. [ Links ]
Timofeeva M., Khankhasaeva S., Badmaeva S., Chuvilin A., Burgina E., Ayupov A., Panchenko V., Kulikova A. (2005). Synthesis, characterization and catalytic application for wet oxidation of phenol of iron-containing clays. Applied Catalysis B. 59: 243-248. [ Links ]
Tunega D., Lischka H. (2003). Effect of the Si/Al ordering on structural parameters ad the energetic stabilizationof vermiculites- a theoretical study. Physics and Chemistry of Minerals 30, 517-522. [ Links ]
Tus¡ar N., Zabukovec N.,Vlaic G., Arc¡on D., Daneu N., Kauc¡ic¡ V. (2005). Local environment of manganese incorporated in mesoporous MCM-41. Microporous and Mesoporous Materials. 82, 129-136. [ Links ]
Weaver C. (1958). Effects ofgeologic significance ofpotassium "fixation" by expandable clay minerals derived from muscovite, biotite, chlorite and volcanic material. American Mineralogist. 43, 839-861. [ Links ]
Wiewióra A., Perez-Rodriguez L., Perez-Maqueda L., Drapala J. (2003). Particle size distribution in sonicated high- ad low charge vermiculites. AppliedClayScience. 24, 51-58. [ Links ]
Wilson M. (1969). Mineralogy: Spectroscopyand Chemical Determinative Methods. Champan & Hall ed; London. [ Links ]