1. Introduction
The world today is facing a crisis due to the lack of clean freshwater. This scarcity of water is a consequence of the rapid development of industries and the large amount of wastewater from industrial processes that is poured into rivers and water systems. This wastewater often contains a great variety of contaminants, many of them in the form of cationic and anionic ions, oils and fats, and other organic residues with harmful effects on ecosystems. Generally, the removal of these contaminants requires effective technologies, which has led to the development in recent decades of cleaning techniques to address this problem.
The rapid economic and industrial growth has brought serious environmental problems, such as air, water and soil pollution. From an environmental point of view, the mining and tanneries sector have always been classified as high polluters, since their production processes produce chemical compounds such as heavy metals and organic waste that causes toxicity and negative environmental impacts in ecosystems. Chromium, for example, is a chemical agent which has been used in the tannery industry because of the high-quality leather it helps to make. Yet when wastewater containing chromium (Cr) is discharged into the environment it damages it. Chromium removal from wastewater is required to prevent water pollution in rivers [1][2] and required to comply with current regulations here in Colombia, such as Resolution 631 of 2015.
Chromium (Cr) is one of the most harmful heavy metals on the environment. Hence the effects of its presence in water and soil as well as the potential solutions have become the subject of intensive research in recent years. Under oxidizing, neutral and alkaline conditions, hexavalent chromium (VI) is present as a chromate or a dichromate. In reducing conditions, the conversion of Cr (VI) to Cr (III) can take place. Contamination by inorganic agents such as chromium has serious consequences for both the environment and the health of those who handle it. Studies carried out by Padma and Dhara (2008) [2] found that wastewater from tanneries carries chromium in a state of oxidation (VI) due to the oxidation of Cr (III), thus contaminating soil and water.
Another type of pollution evident is organic pollution through fat waste and liming among other residual products in the leather manufacturing process. These affect the Tunjuelito River ecosystem in Colombia, thus causing the phenomenon of eutrophication. These pollutants also cause bad odors, due to pipe clogging caused by the accumulation of fats, etc.
Moreover, natural ecosystems such as wetlands are abundant in nutrients, water and sunlight. Due to this, it is common to find the presence of certain types of plants that have developed morphological and biochemical adaptations, which enables them to take full advantage of the conditions in their environment. These plants have been commonly called ‘aquatic weeds’. Among these weeds we find the macrophyte species Eichhornia crassipes, popularly known as the ‘water hyacinth,’ which is widely found in in open bodies of water [4][5].
Through this plant it is possible to build phytoremediation technology to decontaminate water containing chromium [6][7][8][9][10]. In recent years the species has been shown to be capable of sustainable manipulation in its ecosystem and can be used for the phytoremediation of water contaminated with heavy metals [3][11][12][13]. Along these lines, a variety of types of treatment models have been designed, with great efficacy in the removal of contaminated water [14][15][16][17][18][19][20][21].
Bioremediation with this plant is an efficient technology for the treatment of contaminated water and is also a low-cost treatment, since it does not require sophisticated infrastructure [22][23][24][25][26]. It is generally a cheap, simple, sustainable, and environmentally-compatible technology. There are numerous studies worldwide which demonstrate the ability of this plant to remove nutrients, heavy metals and large quantities of organic matter [27][28][29][30][31][32][33]. Studies [1][7][13][34][35]and [16]evaluated various heavy metals such as mercury, aluminum and others. Furthermore, the authors of [36][37][38]and [39]designed this technology for the removal of nutrients in wetlands and obtained significantly positive results.
The main objective of the present project was to design, develop and evaluate new treatment technology for the uptake of chromium by Eichhornia in order to apply this new technology to the tannery sector.
2. Materials and Methods
In this research we employed a quantitative methodology. First we characterized tannery wastewater to establish the degree of contamination. Subsequently, we isolated Eichhornia crassipes from a wetland on the outskirts of the city of Bogota, Colombia, in order to carry out the design and assembly of an Eichhornia phytoremediation system which will treat wastewater contaminated with organic waste and chromium. Finally, we proceeded to the evaluation through a mass balance, comparing the effectiveness of this removal treatment.
2.1 Characterization of the St. Benedict tanneries wastewater
Until now, there had been no assessment of wastewater from these tanneries, which meant that the exact amount of organic load and contamination by chemicals discharged into the sewer was unknown. We visited the tannery sector multiple times to diagnose the problem. The following diagram, Figure 1, shows the production process of a conventional tannery.
We proceeded to characterize the tannery wastewater by quantifying chromium contamination and organic waste. Table 1 shows the parameters carried out by an accredited company. The tests were performed in two tanks, one used for tanning and the other used for liming waste, which holds water contaminated with chromium. These discharges occur daily in this tannery.
As shown in the table above, the amount of chromium discharged daily from the liming tank to the sewer is high: more than 1400 mg/L of chromium is a considerably high amount, since regulations indicate that this figure should be less than 1 mg/L. Resolution 631 of 2015 states:
“Whereby the parameters and maximum permissible limit values are set for specific discharges to water body surfaces and public sewer systems and other provisions .”
The levels of BOD and COD are also very worrying because more is being discharged than expected - 800 mg/L and 1500 mg/L respectively, and there is no control over these discharges. These high rates are the result of fat, blood, and other substances.
This discharge of fat is noticeably very high, a situation which is complicated due to the constant sewer blockages it causes in the area and consequent sewage flooding. The presence of total and fecal coliforms is due to the fact that these tanks are connected to company bathrooms.
2.2 Isolation of Eichhornia crassipes from different wetlands
Eichhornia crassipes was identified in contaminated waters outside the town of Mosquera, Cundinamarca. Figures 2 and 3 show the location were Eichhornia crassipes were found, at coordinates 4.682995, -74.256673.
2.3 Design of phytoremediation technology at a pilot scale using Eichhornia crassipes from water contaminated by the tanneries of San Benito, Southern Bogota
The site is located in the south of Bogota, at the San Benito neighborhood. Figures 2 and 3 show Bogota and the tannery sector.
(Source: Google Maps, 2015)
After the isolation of Eichhornia crassipes, it was brought to the laboratory for the experiments. The proposed design consisted of setting up treatment apparatuses three different times, with 20% tannery water, called 2a, 2b and 2c. The remainder was completed with distilled water. A test target was assembled with natural wetlands water for comparison. Wastewater from the tanneries was collected at a company in San Benito in southern Bogota. Table 2 shows a summary of these setups.
Table 2 Percentages of distilled water and tannery wastewater
| Tanning wastewater (%) | Distilled water (%) | |
| Treatments 2a, 2b and 2c | 20 | 80 |
| Target: 100% water | 0 | 0 |
The design of each treatment apparatus at the pilot laboratory scale consisted in adapting a resistant plastic container with the dimensions shown in Figure 4.
We used two Eichhornia crassipes plants for each treatment, each of which weighted 180g. As seen in Figure 4, the dimensions of this treatment apparatus are: 40 cm in length, 17.5 cm in height and 17.5 cm in width. We also used 11.5 liters of water. This design was made at laboratory-scale.
2.4 Evaluation of the laboratory-scale phytoremediation treatment technology using Eichhornia crassipes
The evaluation of the proposed treatment technology lasted for about one month, as did the experiment in [20]. Hexavalent chromium was measured through spectrophotometry. Table 3 shows the necessary samples for the evaluation of the treatment apparatuses.
Table 3 Sampling water quality
| BOD | Chromium | |
| Treatments 2a, 2b and 2c | Before, and at the end | Every two days |
| Target | Before, and at the end | - |
Samples of Chromium and BOD were taken at the beginning. During the experiment we measured the degree of concentration of chromium in the water every other day, and at the end of the experiment we measured the BOD. We also measured the degree of accumulation of chromium in plants, by taking 180g of the plants to an oven and then separating the absorbed chromium using a filter, separating it from the ashes and then weighing it. The results showed the amount of chromium in mg out of the 180g sample.
3. Results
The evaluation of these phytoremediation treatment systems was performed by determining the pH, the concentration in mg/L of chromium in the water every two days and the BOD before and after treatment. The amount of chromium found in different Eichhornia tissues, especially the stem and root, was also tested. The results were compared with previous research. Three similar treatments were performed, in which the difference between each was negligible. The reliability of these treatment systems was also tested through probability distribution.
3.1 BOD removal tests
We performed two water quality monitoring tests on the BOD in each of the treatments. The tests were performed on Day 0 and after 20 days. Figure 5 shows each of these, with the initial and final concentrations of BOD in mg/L.
The three treatments yielded very similar results, with removals of 80%. The test target removals reached above 83%. The contributions of nutrients to plants and the oxygenation they provide to the water contribute to the reduction of BOD in tannery wastewater. The treatments (2) were at around 1000 mg/L levels of BOD and the test target was at around 1200 mg/L. The experimentation started with a lack of oxygen in the water represented with a very high BOD level, despite dilutions with distilled water. After the treatment we observed some representative decreases, and in treatments 1 and 2 the BOD lowered to 200 mg/L.
3.2 Chrome evaluation
In order to evaluate our treatment technology we measured chromium concentrations in the water in mg/L, at the beginning and then every two days. Figure 6 shows that chromium concentrations were around 612 mg/L for the initial sample on Day 0 of the experiment. Figure 7 shows that after only two days there was a removal of 33%. There was then a continuous removal which stabilized after 24 ways of treatment.
As shown in Figures 6 and 7 there was an initial removal of 34% on Day 1. It then steadied for several days at over 50%. By the end it showed a final removal of more than 70% on these treatments of 20% tannery water and 80% distilled water. The three tests showed a similar behavior throughout the process and removals were obtained at above 70%.
3.3 Mass balance of chromium
At the end of the Eichhornia crassipes treatment process, we tested the aquatic plants for chromium concentration in their vegetative structure. These results show the quantity of chromium in mg in the 180g Eichhornia crassipes sample that was used in the three different experiments.
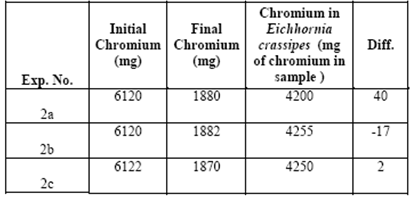
Table 4 shows concentrations of around 4200 (mg chromium / sample) found in the vegetative structure for the three treatments. There were removals because the chromium ended up absorbed within the vegetative structure of the aquatic plant. The reason why the chromium adhered to the plant, or which of its compounds helped (its lignin, cellulose or hemicellulose) is the subject of ongoing research.
Table 4 shows the general balance in each of the experiments of the initial concentrations of chromium, together with the concentrations in the plant and the final chromium concentrations.
In this general balance, we considered the initial and final concentrations and the concentration found in Eichhornia crassipes. For the experiment we had 10 liters of water, a combination of both contaminated water and distilled water. At the beginning there was 612 mg of chromium per liter of water. Multiplying 612 mg/L by 10 liters gives a total of chromium in the treatment system of around 6,120 mg.
Using the same procedure, we determined that in the end there were about 1880 mg of chromium remaining in the water. The differences are almost negligible and can be attributed to the uncertainty of the techniques used.
As shown in Figure 8, there was a significant removal of chromium; Eichhornia crassipes retained a lot of it in its roots and leaves. 70% of the chromium present in the water was removed. This experiment could be potentiated by placing another plant to continue the removals until a permissible level of chromium is reached (one acceptable by the environmental authorities).
4. Conclusions and Recommendations
In order to optimize this process we propose the design of a biological filter using materials made of Eichhornia crassipes, by extracting the cellulose of the plant and by chemically modifying zeolites.
Phytoremediation through Eichhornia crassipes is an alternative for use as a heavy metal and organic matter retainer due to its high ability to remove these contaminants from water. It is easy to use since this plant is very common in this region.
Due to the high chromium concentrations in tanneries in southern Bogota and resulting saturation and accumulation, it is essential to use various aquatic plants in the treatment system. In this research we evaluated the behavior of the plant with these high concentrations and concluded that in order to optimize the process plants should be alternated, in order to get removals of above 90%.
The contributions of nutrients to plants and the adsorption of carbon sources contribute to the reduction of BOD. Tannery wastewater is loaded with organic waste, raising the levels of COD and BOD, which makes this treatment technology interesting for further research that might optimize the process.
We recommend a cost study evaluating this treatment in the market of conventional treatment plants for use in the tannery sector, in order to determine the feasibility of this treatment.