1. Introduction
The harmful use of ethanol, defined by the WHO (2018) as a regular intake of 20 to 40 gr daily of the substance in women and 40 to 60 gr in men, ranks third among the main risk factors for premature death and disabil ity worldwide. Harmful use of ethanol resulted in an estimated 3 million deaths (5.3% of all deaths) globally (PAHO, 2020; WHO, 2018).
Ethanol changes the release of GABA, dopamine, en dogenous opioids, and other neurotransmitters in nuclei of the reward system involved in the hedonic effects of substances of abuse (Dharavath et al., 2023; Ericson et al, 2003; Pautassi et al., 2010). These neuroadaptations cause motivational learning and memory processes that can result in the transition from moderate to excessive ethanol (Dharavath et al., 2023; Ron & Barak, 2016).
However, ethanol use may be predominantly moti vated not only by the neurobiological reinforcing effects, but also by its ability to counteract stress and decrease anxiety and depressive emotional states (Koob, 2006; Koob & Le Moal, 2008). These conditions are frequently related to the generation of addictive behavior to ethanol consumption (Briand & Blendy, 2010; Kushner MG, 2000; Skelly et al., 2015). Although, the accumulated evidence is not conclusive in the relationship between anxiety states and ethanol consumption in rats (Acevedo et al., 2014; Peregud et al, 2021; Popovic et al., 2004; Rimondini et al., 2003).
Anxiety can be evaluated through different experi mental models, such as the elevated plus maze, which is based on the natural choice for closed and dark spaces, when exposed animals to the maze avoid opposite spaces, it is a measure of anxiety state (Peregud et al., 2021; Van Skike et al., 2016).
In the animal facility of the Faculty of Health-UIS, a special strain of Wistar rats is housed. These inbreed an imals have been possible to characterize, behaviorally, in two strains: “Reactive” and “Non-Reactive”. These two strains are characterized by having a differential anxi ety behavior: the “Reactive” strain presents an increase in anxious behaviors (low entries to open arms on the elevated plus-maze); while the “Non-Reactive” strain ex poses a decrease in this type of behavior (high entries to open arms on the elevated plus-maze) (Baez, 2001). With these anxiety-related behaviors of the strains, our hypothesis was that the voluntary ethanol consumption increases the anxious-related behavior on the Wistar- UIS strains. Therefore, the aim of the present study was to evaluate Wistar-UIS strains’ anxiety-related be haviors before and after the voluntary consumption of ethanol (10% v/v) protocol.
2. Materials and Methods
2.1 Animals
The study was conducted with a total of 37 male Wistar- UIS rats, with approximate ages of 2 months and weights greater than 200 gr. The animals were divided into three behavioral groups: Wild-type (W, n = 13), Reactive (R, n = 12), and Non-Reactive (NR, n = 11). All animals were housed in individual boxes with food and water ad libitum, with a light-dark cycle of 12 hours (7-19), with controlled temperature and humidity (21 ± 2°C; 65% ± 5). The conditioning of the individual housing was carried out for one week before the start of the con sumption protocol.
The experiments were carried out with the approval of the ethics committee of the Universidad Industrial de Santander (COIE).
2.2 Protocol of voluntary and intermittent consump tion of 10% ethanol
For habituation to the housing conditions, all groups had at their disposal two bottles with 200 ml of water for 7 days. Before starting the ethanol consumption protocol, the animals were exposed to a mixture of .1% (v / v) of ethanol to avoid neophobia before the higher percentage of ethanol consumption. Subsequently, the protocol of in termittent consumption of 10% ethanol (v / v) consisted of the exposure of a bottle of water and a bottle with 10% ethanol. The ethanol bottle was placed on Mon days, Wednesdays, and Fridays. During the weekends, all the animals received two bottles of water. To cal culate the consumption, all bottles were weighted in all groups after 24 hours of exposure and the animals were weighted at the end of each week. Therefore, the water or ethanol consumed in 24 h (gr) per animal weight (kg) (g/kg/24 h) were taken as consumption per day. This protocol was adapted from Simms et al. (2008).
2.3 Elevated Plus-Maze
The Elevated Plus-Maze (EPM) is a wooden device with 4 arms of 50 x 12 cm arranged in the form of a cross and raised to 50 cm above the ground. Two of its arms, the so-called open, have as side walls a small acrylic border 2 cm high except in its communication with the center of the labyrinth. The so-called closed arms have wooden side walls 40 cm high, except in their communication with the center of the labyrinth. The animals were ex posed to EPM for 5 minutes to evaluate behaviors re lated to the state of anxiety. The sessions were moni tored and filmed with the help of a digital video camera placed approximately 1.5 meters above the maze; its sig nal was sent to a computer that is in a room adjacent to the experimentation room. The number and percentage of entries to the open arms (OA), and their locomotor activity, were recorded and evaluated. These records were made with the help of a set of computer programs developed for this type of experiment (Conde C, 2000). Two exposures were carried out: before the start of the consumption protocol and 24 hours after the last expo sure to the ethanol bottle (day 40).
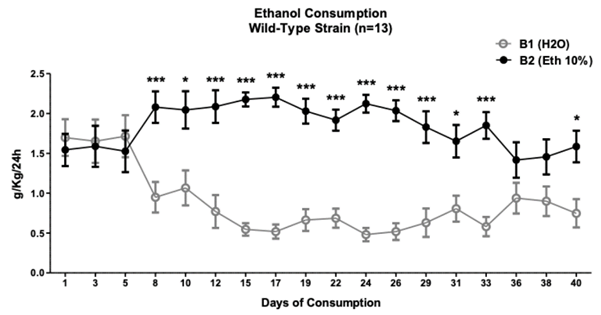
Note. It shows the days and the amount of consumption (g/kg/24 h), where days 8 to 33 and day 40 of ethanol consumption were higher than water consumption. The circles show the mean ± SEM at each day. *p < .05, ***p < .001
Figure 1 Ethanol Consumption in Intermittent Exposure Protocol in Animals of the Wild-type Wistar-UIS Strain
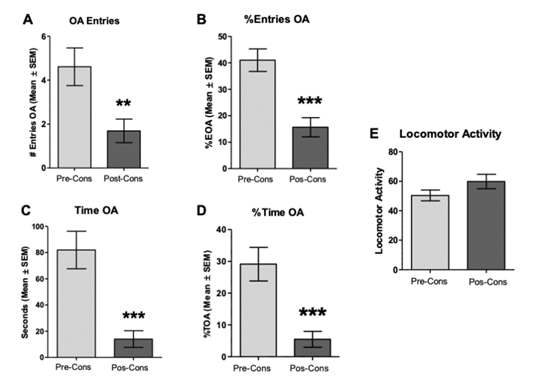
Note. A. number of entries to the OA of the EPM pre- and post-protocol of intermittent consumption of ethanol, where a decrease of these is shown after exposure to consumption. B. OA entries in the EPM. C. time within the BA of the EPM. D. relative time in the OA of the EPM. E. Locomotor activity is evidenced in the crossings made between the different places of the EPM. All figures show the mean ± SEM. **p < .01, ***p < .001
Figure 2 Exposure to pre- and post-consumption LCE of Ethanol in the Protocol of Intermittent Consumption of 10% Ethanol in the Wild-type Strain Wistar-UIS (n = 13)
2.4 Statistical Analysis
Statistical analysis was performed using the GraphPad Prism program (GraphPad, San Diego, CA). Data (g/Kg /24 h) of ethanol consumption for each strain group were analyzed using two-way repeated measures ANOVA on each recorded variable, followed by multiple compar isons applying the Bonferroni posthoc. The factors that were evaluated were factor 1: the contents of the bottle (water or ethanol); factor 2: the day of consumption.
The data recorded from the EPM were crossings, number, and percentage of entries to open arms. Com parisons of these data were made between sessions of each of the strains using the t-paired test. The signifi cance level was set at 5% for all cases.
3. Results
3.1 Ethanol Consumption in Wild-Type Strain
The consumption of 10% ethanol through the protocol of intermittent exposure in animals of the Wild-type strain shows the beginning of the preference with sig nificant differences for the bottle with ethanol (2.08 ± 0.19 g/kg/24 h) compared to the bottle of water (.94 ± .19 g/kg/24 h) from day 8 (fourth day of exposure to the bottle with ethanol) (two-way repeated measures ANOVA (F (17, 26) = 6.463), p < .0001; content factor p < .0001, time factor p = .2). Bonferroni posthoc showed this significant difference until day 33 and 40 (p < .01) (See Figure 1).
3.1.1 Effect of ethanol consumption on anxiety behavior in the Wild-type strain
To demonstrate the effect of ethanol consumption on anxiety-like behavior, the animals were exposed to EPM for 5 minutes the day before starting the protocol of in termittent ethanol consumption and to a second session 24 hours after the last exposure to the water and ethanol bottles’ (day 41). The comparison made with t-paired tests shows significant differences between the pre- and post-consumption sessions in the number of entries to the OA (t = 4.21, df = 12, p < .01), entries relative to the OA (t = 7.81, df = 12, p < .0001), time in the OA (t = 5.31, df = 12, p < .001), and relative time in the OA (t = 5.30, df = 12, p < .001) as shown in Figure 2A, 2B, 2C and 2D, respectively. On the other hand, there were no significant differences in the locomotor activity of these animals between the pre- and post-consumption LCE session (t = 1.85, df = 12, p = .08; Figure 2 E). These results indicate that exposure to ethanol in the inter mittent consumption protocol did not present anxiolytic effects in animals of the wild-type strain.
3.2 Ethanol Consumption in Reactive Strain
The consumption of 10% ethanol in the intermittent pro tocol in the Reactive strain shows a change in preference for the bottle with ethanol towards day 10 (1.67 ± .14 g/kg/24 h) compared to the bottle with water (.80 ± .13 g/kg/24 h) with a significance of p < .01. This signifi cant difference is maintained during days 12, 15 and then from day 22 to 36 and finally occurs on day 40 (two-way repeated measures ANOVA F (17.22) = 5.58; content fac tor p < .001; day factor p = .98; interaction p < .0001). Bonferroni posthoc showed this significant difference on day 10 to 15, 22 to 31, 36 and 40 (See Figure 3) .
3.2.1 Effect of ethanol consumption on anxiety behavior in the Reactive Strain
The results of the behavior within the EPM of animals of the Wistar-UIS Reactive strain show that in none of the variables evaluated were found significant differences: entries to OA (t = 1.23, df = 11, p = .24; see Figure 4 A), percentage of entries to OA (t = .24, df = 11, p = .80; see Figure 4 B) , time in OA (t = 1.20, df = 11, p = .32; see Figure 4 C) , percentage of time in OA (t = .71, df = 11, p = .48; see Figure 4 D), and locomotor activity (t = .38, df = 11, p = .70; see Figure 4 E). The present results indicate the state of anxiety was not modified by the consumption of ethanol in the Reactive Strain.
3.3 Ethanol Consumption in Non-Reactive Strain
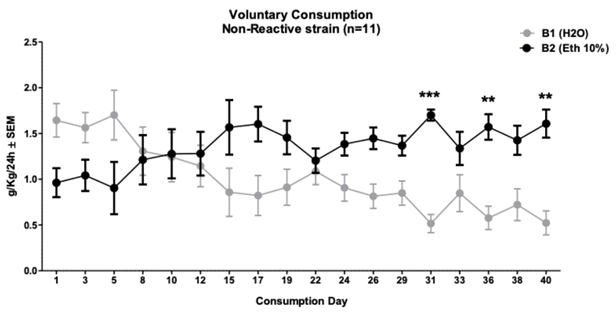
The protocol of intermittent consumption of 10% ethanol in animals of the Non-Reactive strain (n = 11) showed significant differences (p < .0001) late on day 31 between water consumption (.51 ± .09 g/kg/24 h) and ethanol (1.7 ± .06 g/kg/24 h). Also, on days 36 and 40, they presented significant differences (two-way repeated mea sures ANOVA F (17, 20) = 6.48; content factor p = .03, day factor p = .93, interaction p < .0001). Bonferroni posthoc showed this significant difference until on day 31, 36 and 40 (See Figure 5).
3.3.1 Effect of Ethanol Consumption on Anxiety Behavior in the Non-Reactive Strain
The results of the behavior within the LCE of animals of the Non-Reactive strain Wistar-UIS show that in none of the variables evaluated, there are significant differences: number of entries to OA (t = .1474, df = 8, p = .88; see Figure 6 A), percentage of entries to OA (t = .3012, df = 8, p = .77; see Figure 6 B), time in OA (t = 1.193, df = 8, p = .26; see Figure 6 C), percentage of time in OA (t = 1.171, df = 8,p = .27; see Figure 6 D), and locomotor activity (t = 1.273, df = 8, p = .23; see Figure 6 E). The present results indicate the absence of anxious behavior on animals of this strain after chronic consumption of ethanol.
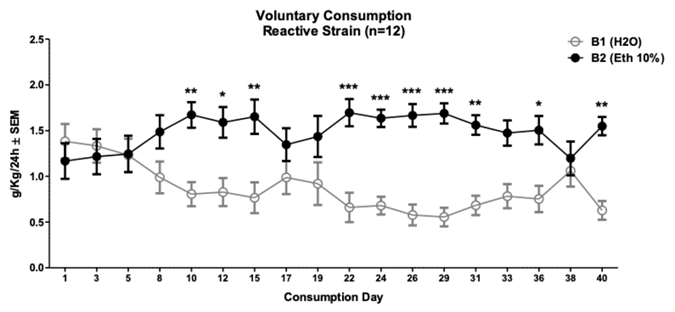
Note. It shows the days of consumption and the amount (g/kg/24 h), where days 10, 12, 15, 22 to 36 and day 40 of ethanol consumption were higher than water consumption. *p < .05, **p < .01, ***p < .001
Figure 3 Consumption of 10% Ethanol in the Intermittent Protocol in Animals of the Wistar-UIS Reactive Strain (n = 12)
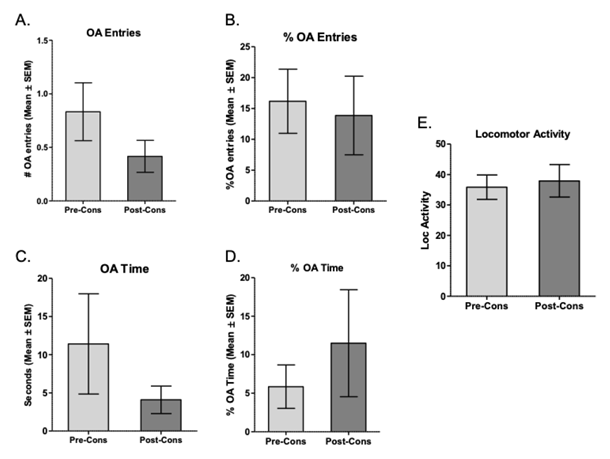
Note. A. number of entries to the OA of the LCE pre- and post-protocol of intermittent consumption of ethanol. B. Percentage of OA entries in the LCE. C. Time within the OA of the EPM. D. percentage of time in the OA of the EPM. E. Locomotor activity is evidenced in the crossings made between the different places of the EPM. All figures show the mean ± SEM
Figure 4 Exposure to pre- and post-consumption EPM of Ethanol in the Protocol of Intermittent Consumption of 10% Ethanol in the Wistar-UIS Reactive Strain (n = 12)
Note. It shows the days of consumption and the amount (g/kg/24 h), where days 31, 36, and 40 of ethanol consumption was higher than water consumption. The circles show the mean ± SEM at each day. **p < .01, ***p < .001
Figure 5 Consumption of 10% Ethanol in the Intermittent Protocol in Animals of the Non-Reactive Wistar-UIS Strain (n = 11)
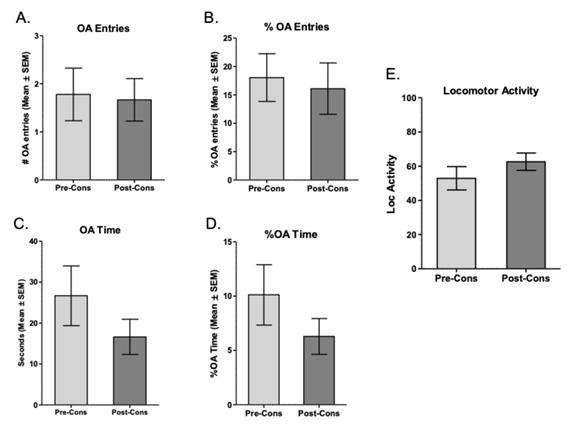
Note. A. number of entries to the OA of the EPM pre- and post-protocol of intermittent consumption of ethanol. B. Percentage of OA entries in the LCE. C. Time within the OA of the EPM. D. Percentage of time in the OA of the EPM. E. Locomotor activity is evidenced in the crossings made between the different places of the EPM. All figures show the mean ± SEM
Figure 6 Exposure to pre- and post-consumption EPM of Ethanol in the Protocol of Intermittent Consumption of 10% Ethanol in the Wistar-UIS Non-Reactive Strain (n = 11)
4. Discussion
The present work shows the change in ethanol (10% v/v) preference over water in a protocol of voluntary intermit tent consumption, in the three groups of animals sub jected to this protocol. Additionally, it shows how the ethanol preference may be decreasing the presentation of signs of anxiety in animals with behaviors characterized as anxious, as it is in the Reactive and Non-Reactive strains that are housed in the Animal Facility of the Industrial University of Santander.
The development of ethanol consumption depends on multiple factors such as the genetic predisposition of individuals, previous and related experiences with con sumption, and social inducers, among others (Bell et al., 2016; Goodwin et al., 2000; Pautassi et al., 2010).
In general, in the present study, the use of the proto col of intermittent voluntary consumption showed that consumption levels were like low-consumption animals shown in other studies (2-4 g/kg/day) (Carnicella et al 2014; Simms et al., 2008; Wscieklica et al., 2019). How ever, even though consumption is low, it is possible to see a marked preference between the consumption of wa ter and ethanol, ending with the latter as preferential in all strains. Different studies had shown Wistar rats has lower ethanol consumption on the intermittent-access ethanol drinking protocol (1 to 5 g/kg/day) in compari son to Long-Evans rats (Carnicella et al, 2014; Simms et al., 2008). Also, Sprague-Dawley rats have shown higher ethanol consumption with this paradigm (3-5 g/kg/day) (Carnicella et al, 2014). This study indicates that de spite low ethanol consumption on Wistar-UIS rats, there is a preference compared to water consumption.
4.1 Ethanol Consumption and Anxiety Behavior on Wild-Type Strain
In the present work, we wanted to verify that the con sumption of chronic ethanol is interrelated with anxi ety states, reaching to modify them. In the case of Wild-type Strain, which does not present specific char acteristics of anxiety-related behaviors, it was observed that after intermittent ethanol consumption, the ani mals showed a decrease in exposure (entries and time) to the open arms of the EPM. This indicates that ethanol consumption increased anxiety-related behaviors on these animals. Similar results were described by Van Skike et al. (2015), where they showed that intraperitoneal ethanol injection chronically increased anxious behavior, with decreased entries and permanence to open arms of the EPM in young and adult animals. Also, chronic in termittent ethanol vapor exposure protocol has been re ported to increase anxiety-related behaviors on the EPM on male rats (Ewin et al., 2019; Morales et al., 2018). These findings are important because not only ethanol consumption can lead to increases in anxious behav iors, but the withdrawal of addictive substances, such as ethanol, can generate the development and exacerbation of these behaviors. Therefore, the interrelation between ethanol consumption and anxiety behaviors leads to the return of uncontrolled consumption of addictive sub stances. These changes in animal behavior can be gen erated by the modification of signaling and neurotrans mission of structures such as the hipp ocampus, amyg dala, hypothalamic-pituitary axis, mesencephalic struc tures, and cortex, among others (Dharavath et al., 2023; George et al., 2012; Koob & Volkow, 2016; Marballi et al., 2016; McCool, 2011; Mrejeru et al., 2015; Sanchez- Catalan et al., 2014; Van Skike et al., 2015; Wscieklica et al., 2019). Regarding changes in neurotransmission, studies show modifications in several systems: gluta mate, GABA, dopamine, corticotropin-releasing factor, and endocannabinoids, among others (Adermark et al., 2011; Cippitelli et al., 2012; Dharavath et al., 2023; Kim & Souza-Formigoni, 2013; Melis et al., 2009).
4.2 Ethanol Consumption and Anxiety Behavior on Reactive Strain
The literature indicates that alcoholism and anxiety are pathologies of high comorbidity, but their direct rela tionship is not yet fully understood. In the case of our Reactive Strain, its preference for ethanol consumption was evident compared to Wild-type Strain, indicating that its anxious condition may influence higher ethanol consumption. EPM exposure in these anxious animals, after ethanol consumption protocol, showed that there were no significant changes, so the preference for ethanol consumption maintained the baseline anxiety levels of these animals. This indicates that chronic ethanol con sumption goes hand in hand with behavioral manifes tations of anxiety, being consistent with the hypothesis that high levels of baseline anxiety generate high volun tary ethanol consumption (Chappell et al., 2013; Izidio & Ramos, 2007; Van Skike et al., 2015).
4.3 Ethanol Consumption and Anxiety Behavior on Non-Reactive Strain
On the other hand, the Non-Reactive Strain, which is characterized by low baseline levels of anxiety, shows a preference for ethanol consumption late (day 31 of con sumption). Such ethanol consumption does not gener ate change in anxious behaviors; therefore, low baseline levels of anxiety can prevent the preference for ethanol consumption in animals with these characteristics and such consumption would not generate high anxious states. Studies show that the reduction of anxiety states, through inhibition of noradrenergic signaling, presents a decrease in ethanol consumption (Munier et al., 2023; Skelly & Weiner, 2014; Varodayan et al., 2022). Our results are con sistent: animals with low anxiety (Non-Reactive strain) show low ethanol consumption. It also indicates that in these animals noradrenergic signaling may be impaired compared to animals of the other strains, so future studies should be conducted to elucidate these differences within our strains. Elucidating the different modulations gener ated by the consumption of ethanol chronically can help increase the possibility of treatments and pharmacologi cal management for the reduction of adverse effects such as anxiety in patients with alcoholism.















