INTRODUCTION
Predatory mites of the Mesostigmata order represent potential biological control agents for agricultural pests (Carrillo et al., 2015). Previous studies have found species of the families Laelapidae and Parasitidae in soils of bulb onion (Allium cepa L.) crops in the Department of Boyaca (Castro-Lopez, 2018), including Gaeolaelaps aculeifer (Canestrini) (Laelapidae), which is used commercially in European and North American crop production for controlling thrips, mites, and flies (van Lenteren, 2012). Likewise, Parasitus bituberosus (Karg) (Parasitidae) is a promising species for managing fly larvae, (Szafranek et al., 2013) thrips, and nematodes (Rueda-Ramírez et al., 2019).
The bulb onion is one of the more important vegetables worldwide (Pinzón, 2009). The presence of Thrips tabaci Lindeman (Thysanoptera: Thripidae) in bulb onion crops is a challenge for production (Vergel et al., 2017) because it reduces bulb size by up to 60% (Rueda et al., 2007). This insect reduces the photosynthetic capacity by feeding on onion leaves (Jensen et al., 2003), accelerating leaf senescence with the physiological stress that results from the foliar damage (Kendall and Bjostad, 1990). It is also a vector of the Iris yellow spot virus (Bunyaviridae: Tospovirus), which reduces bulb size and quality (Muñoz et al., 2014).
Management strategies for T. tabaci include the application of chemically synthesized products. However, T. tabaci populations have been reported to be resistant to several insecticides, including organophosphates, carbamates and pyrethroids in the United States (Shelton et al., 2006), spinosad in Israel (Lebedev et al., 2013), and deltrametrin and diazinon in New Zealand (Martin et al., 2003). In South America, resistance to these chemicals is unknown. In addition, not all insect stages of development are equally affected by chemical treatments (Damte et al., 2017). Prepupae and pupae are protected by the soil or the base of onion plants and may escape contact with insecticides (Gill et al., 2015). Therefore, management strategies that include biological control should be explored.
Gaeolaelaps aculeifer is an edaphic mite that is used commercially for biological control in Europe and North America. This mite is a generalist predator that can feed on a wide range of prey, including astigmatic mites (Rueda-Ramírez et al., 2018), nematodes (Moreira and Moraes, 2015), fly larvae (Sardar and Murphy, 1987), and collembolans (Heckmann et al., 2007). A Colombian population of this predator was found to consume approximately 2.6 Frankliniella occidentalis (Pergande) pre-pupae or pupae daily per adult female (Rueda-Ramírez et al., 2018). In bean plants, a predation rate of 80.5% for F. occidentalis was reported for 14 d (Berndt et al., 2004); in citrus, consumption reached for 12.3±0.8 Pezothrips kellyanus (Bagnall) over 3 d (Navarro-Campos et al., 2012). There are no studies on this predatory mite for T. tabaci.
Parasitus bituberosus is another edaphic mite that has been reported as a potential biological control agent, feeding on fly larvae (Al-Amidi and Downes, 1990; Al-Amidi et al., 1991; Szafranek et al., 2013), nematodes (Szafranek et al., 2013; Rueda-Ramírez et al.,2019), and forpigarous mites (Szafranek et al., 2013). Recently, a study observed the consumption of 2.5 ±0.8 F. occidentalis pre-pupae and pupae daily per adult female (Rueda-Ramírez et al., 2019). This result indicated that this predator may prey on other thrips species, including T. tabaci.
Given these findings and the fact that pre-pupae and pupae stages of T. tabaci develop in the soil, the objective of this research was to evaluate the response of A. cepa plants to applications of G. aculeifer and P. bituberosus to control T. tabaci under greenhouse conditions.
MATERIALS AND METHODS
Geographical location of the study area
This research was carried out in a greenhouse at the Grupo Manejo Biologico de Cultivos (GMBC), Universidad Pedagógica y Tecnológica de Colombia (UPTC), in the municipality of Tunja, Department of Boyaca, Colombia, with an average daily temperature of 21±3ºC and a relative humidity of 60±15%.
In the greenhouse, 60 A. cepa onion seedlings, acquired in the municipality of Tibasosa in the Department of Boyaca, were seeded. Six plants were placed in a 1 m2 square. Conventional management was used for the irrigation, fertilization, and other agronomic tasks to achieve good development of the plants.
The mite colonies were kept in breeding units, which consisted of an incubator chamber with a 19±3ºC average temperature and 60±15% relative humidity, in the absence of light. This methodology was adapted from Freire and Moraes (2007). Gaeolaelapsaculeifer was fed a mixture of all developmental stages of Aleuroglyphus ovatus (Troupeau) (Sarcoptiformes, Astigmatina, Acaridae), which was reproduced and reared on crushed commercial dog food (Purina®; nutritional content: 9% fat, 12% moisture, 8% ash and 25% protein). Parasitus bituberosus was fed with A. ovatus and Rhabditidae nematodes maintained on bean pods (Phaseolus vulgaris L.).
Evaluation of G. aculeifer and P. bituberosus for the control of T. tabaci in onion plants
The onion plants were infested with 50 T. tabaci adults that were collected from A. cepacrops in the municipalities of Tibasosa and Nobsa. After 60 d, they were expected to have completed four life cycles. Afterwards, ethe prepupae and pupae were used as food for the predatory mites; different amounts of the mite predator species were released.
A completely randomized experiment design was used that included seven treatments: 50 (G50), 75 (G75), or 100 (G100) f G. aculeifer adults, 50 (P50), 75 (P75), or 100 (P100)P. Bituberosus adults, and a control without applications of the predator (control). Each treatment was replicated six times, for a total of 42 experiment units. Two releases of G50, G75, G100, P50, P75 and P100 were carried out for the treatments, at the beginning of assay and in the 8th week.
Every 2 weeks, the T. tabaci population was assessed, recording all developmental stages (excluding the eggs of the thrips) found on three leaves in the central third of six plants per treatment. This method was used from the 5th week until the 13th week,which was the end of the growing cycle.
Effects on bulb onion plants from the G. aculeifer and P. bituberosus
To determine the effects on onion plant growth, five leaves were selected from the central third of each plant and treatment to measure the chlorophyll content (Minolta SPAD 502 plus chlorophyllmeter) every 15 d, recording the average per plant, expressed in SPAD Units. At week 14, when maturity was reached, the foliar area was measured, expressed in cm2 were done, with a CI 202, Bio-Science Inc. The fresh and dry plant weights were quantified using an electronic balance (Acculab VIC 612 - 0,01 g precision). The plants were dried for 48 h at 70ºC in a Memmert oven.
In addition, soil samples were taken with a metal cylinder,10 cm diameter and 5 cm depth. The samples were transported in plastic bags to the laboratory, where the mites were extracted with a modified Berlesse funnel (Oliveira et al., 2000). Each sample was observed with a stereomicroscope (Leica microsystems) to confirm the presence of the Mesostigmata mites. The variables were measured in the laboratories of Biological control and Plant physiology of the UPTC.
The data were subjected to normality and variance homogeneity tests using Shapiro-Wilk and Bartlett tests, respectively. When the assumptions were verified, the variables that had statistical differences were tested with Tukey’s comparison of means (P≤0.05). The analyses were carried out in R (version 3.3.2).
RESULTS AND DISCUSSION
Population estimate of T. tabaci in onion plants with G. aculeifer and P. bituberosus
The population of T. tabaci in the onion plants declined, with significant differences (P≤0.05) between the six treatments with G. aculeifer(G50, G75, and G100,) and the control after week seven. The Tukey test corroborated the differences, with 10±3.6; 26±4.3 and 17±2.6 T. tabaci individuals on the leaves, which indicated a 78, 63 and 48% population decrease for the treatments with G100, G75 and G50 G. aculeifer, respectively (Fig. 1A).
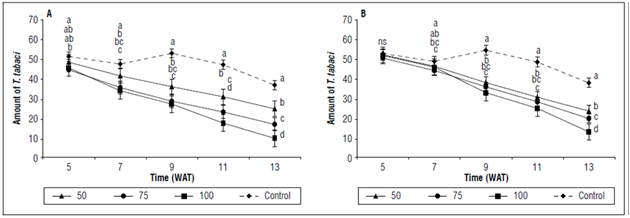
Figure 1. Population density of Thrips tabaci in bulb onion plants (A. cepa) exposed to different concentrations of the predatory mites G. aculeifer (A) and P. bituberosus (B) and a control without application for 13week after transplant (WAT). Means with different letters indicate a significant statistical difference, and ns indicates no statistical difference according to the Tukey test (P≤0.05). The vertical bars indicate ± standard error.
A similar response was observed in the onion plants with P. bituberosus after the 9th week, with counts of 13±2.0; 23±2.4 and 19±2.6 individuals, indicating a reduction of 72, 62, and 55% in the T. tabaci population, as shown in figure 1B.
Our results showed a reduction of 48-78% in the T. tabaci population withG. aculeifer and P. bituberosus over 13 weeks. This was comparable to reports for other species of edaphic mesostigmatid mites. For example,Stratiolaelaps scimitus (Womersley) reduced the population density of T. tabaci by64% over 6 weeks (Wu et al., 2014), Hypoaspis miles (Berlese) saw a reduction of 44.9%, G. aculeifer (mentioned as Hypoaspis aculeifer) saw a reduction of 57.6% for 14 d (Berndt et al., 2004) and 51%, and Macrocheles robustulus (Berlese) saw a reduction of 66-70% for 13 weeks (Messelink and van Holstein-Saj, 2008), feedingon F. occidentalis. However, the edaphic phases ofT. tabacicontrol only provide an alternative within integrated management programs because approximately 20-25% of thrip populations can complete their life cycle in the presence of predatory mites (Berndt et al., 2004).
These differences in the predation rate may be related to several factors, e.g. slight contortions that have been observed in the pupal stages of thrips (Rueda-Ramírez et al., 2019), size of the pre pupa and pupa, which are usually smaller (0.91-0.96 mm) (Shaikh et al., 2015) than F. occidentalis (1.1-1.3 mm) (Cárdenas and Corredor, 1989). This may determine the preference of G. aculeifer and P. bituberosus for some types of prey.
Effects on bulb onion plant development with G. aculeifer and P. bituberosus
Evaluation of the relative chlorophyll index showed statistical differences (P≤0.05) between the treatments after the 7th week, when the bulb formation phase began (Fig. 2). The treatment with 100 individuals of predatory mites presented the highest value for this index in week 13, with 135±3.4 SPAD units.
The results in figure 2 indicated that the onion plants infested with G. aculeifer and P. bituberosus had a higher chlorophyll content than the control plants (97±2.0 SPAD units), and the treatment with 50 P. bituberosus individuals achieved 103±3.2 SPAD Units within 13 weeks, possibly because a small number of P. bituberosus mites preferred another type of prey rather than thrips, such as small insects, collembolans and other mites that live in the soil (Castilho et al.,2015).

Figure 2. Relative chlorophyll index SPAD in bulb onion plants (A. cepa) exposed to different concentrations of the predatory mites G. aculeifer (A) and P. bituberosus (B) and a control without application in the 13 th week after transplant (WAT). Means with different letters indicate a significant statistical difference, and ns indicates no statistical difference according to the Tukey test (P≤0.05). The vertical bars indicate ± standard error.
The leaf area showed significant differences between the treatments. According to the Tukey test (P≤0.05), this result was more evident with the treatments where G. aculeifer was added, with an average leaf area for all treatments of 238±14.7 cm2, while this average for P. bituberosus was 220±9.7 cm2. Both were higher than the control (130±16.8 cm2)(Fig. 3).

Figure 3. Leaf area in bulb onion plants (A. cepa) exposed to different concentrations of the predatory mites G. aculeifer and P. bituberosus and a control without application. Means with different letters indicate a significant statistical difference according to the Tukey test (P≤0.05). The vertical bars indicate ± standard error.
Pineda-Mares et al. (2001) indicated that leaf lamina is one of the most important parts for developing photosynthesis. T. tabaci adults and individual nymphs that perforate the surface tissues to absorb cellular contents interfere with this process (Jensen et al., 2003), directly affecting leaf area (Marschner, 2012). Likewise, the populations of T. tabaci were significantly higher when the crop had a growing period longer than 3 weeks, resulting in longer leaves that can be more attractive for thrips than in seedlings (Hsu et al., 2010). This behaviour was observed here, especially in the control plants.
The dry leaf weight had significant differences between the treatments (P≤0.05). The higher values were 52.9±2.29 and 55.2±2.59 g when 100 individuals of G. aculeifer or P. bituberosuswere added, respectively (Fig. 4). In comparison, the control treatment presented the lowest value with 28.2±1.78 g, indicating a negative effect when onion plants do not have any control for T. tabaci because the nutrient supply is directly related to the accumulation of dry matter (Hernández et al.,1996).

Figure 4. Dry leaf weight in bulb onion plants (A. cepa) exposed to different concentrations of the predatory mites G. aculeifer and P. bituberosus and a control without application. Means with different letters indicate a significant statistical difference according to the Tukey test (P≤0.05). The vertical bars indicate ± standard error.
A decrease in the dry matter was observed in figure 4, indicating that there was a decrease in the uptake and efficiency of radiation, as reflected in the accumulation of dry matter in the onion plants. Since this parameter indicates the amount of photoassimilates stored in different parts for plant growth (Estrada-Ortiz et al., 2011), directly affecting production, conversion of simple sugars in leaf tissue and bulb growth (Dogliotti et al., 2011) (Fig. 5).

Figure 5. Fresh bulb weight (g) in bulb onion plants (A. cepa) exposed to different concentrations of the predatory mites G. aculeifer and P. bituberosus and a control without application. Means with different letters indicate a significant statistical difference according the to Tukey test (P≤0.05). The vertical bars indicate ± standard error.
In terms of fresh bulb weight, there were significant differences between the treatments (P≤0.05). The higher weights were 1,341.15±29.1 g and 1,188.36±12 g with the presence of 100 individuals of G. aculeifer or P. bituberosus, respectively. Additionally, the weight with the release of 50 P. bituberosus mites (618.3±16.2 g) was remarkably similar to the control (620.1±29.3 g) (Fig. 5) because the number of soil predatory mites was not sufficient for managing T. tabaci, affecting the fresh bulb weight.
The chlorophyll content is directly related to the photosynthetic rate (Shekari et al.,2017), so, direct damage by T. tabaci destroys the mesophyll, affecting the amount of chlorophyll in the leaf (Molenaar, 1984) and interfering with the transport of nutrients to the bulb (Parrella and Lewis, 1997; Diaz-Montano et al., 2011). On the other hand, a higher production of ethylene is induced in onion plants when the salivary gland products of T. tabaci affect tissues (Kendall and Bjostad, 1990), inducing early maturation and senescence of the leaves (Levy and Kedar, 1970). This was observed in the present study because the control had the lowest values for the evaluated variables, such as leaf area, leaf dry weight and fresh bulb weight, as reflected in the growth and development of the onion plants.
Despite the physiological differences obtained with the predatory mite releases evaluated in the onion plants, alternative management strategies are recommended to suppress populations of T. tabaci because combined strategies result in more efficient insect pest control. For example, S. scimitus and Gaeolaelaps gillespiei (Beaulieu) (Mesostigmata: Laelapidae), when used with Metarhizium anisopliae (Metschnikoff) and Beauveria bassiana (Balsamo), achieved a mortality greater than 90% for F. occidentalis (Saito and Brownbridge, 2016). These components may be considered for future research and field verification, especially for the protection of bulb onion leaves, where juvenile and adult T. tabaci are found, playing an important role in reducing yield by damaging crops (Jandricic et al.,2016).
CONCLUSIONS
The results in this research indicated that releasing the predatory mites G. aculeifer and P. bituberosus reduced the population of T. tabaci by 72 and 78%, respectively. Consequently, the relative chlorophyll index, leaf area index, dry leaf weight and fresh bulb weight increased when compared to the onion plants without a control for this bulb onion insect pest.
The highest values in the physiological variables, which contribute to the yield of bulb onion crops, were obtained with the release of 100 individuals of G. aculeifer or P. bituberosus, representing a new tool for integrated management programs for T. tabaci.





![Morphometry, viability and germination of seeds of Benincasa hispida [(Thunb.) Cogn.]](/img/en/next.gif)









