INTRODUCTION
Worldwide, one of the greatest public health concerns is the increase in the appearance and spread of microbial pathogens (Malik et al., 2017); in developed countries, half of deaths are due to infectious diseases for which treatment with existing drugs is not enough because of common pathogen resistance (Rodríguez et al., 2017). In the food industry, there have been high degrees of toxicity and carcinogenicity reported for synthetic additives used for food preservation (Khalil et al., 2018).
This problem has a high impact on life, in addition to the cost-benefit of the treatments to treat this pathology, which affects all countries (García-Perez et al., 2021). According to a report by the National Institute of Health (Colombia Instituto Nacional de Salud, 2022), in Colombia, there is an annual increase of 33% in food-borne disease outbreaks compared to the previous years; where diarrheal diseases are the ones with the highest incidence transmitted by microorganisms such as Salmonella sp., Campylobacter, and Escherichia coli ([Migula] Castellani and Chalmers).
This has led to the search for new alternatives, where bioprospecting and the use of natural resources are fundamental tools. Plants have the ability to produce a variety of secondary metabolites with significant antibacterial activity that could break the resistance generated by synthetic antibiotics and efficient in the treatment of bacterial infections (Torres-Chati et al., 2017; Palacios et al., 2019; Mosquera et al., 2020).
In recent years, studies of the Annonaceae family have intensified because are sources of important phytochemical agents with broad potential (Krinski et al., 2014; Giraldo-Rivera and Guerrero-Alvarez, 2019; León-Méndez et al., 2020). Annonaceae is a family of lowland trees that grow mainly in forest and lianas and is found mainly at elevations below 2,000 m. This family has 130 genera, and it is estimated that there are about of 2,450 species in the world, distributed throughout the tropical zones of America, Africa, Indochina and Malaysia; among them there are numerous fruit trees, especially in the genera Annona and Rollinia. It has four genera of economic interest in fruit growing: Annona, Rollinia, Uvaria and Asimina (Maas et al., 2019).
The genus Annona is the second-largest genus of Annonaceae in the Neotropics with a total number of 160 species and the most used are Annona cherimola (Miller) (chirimoya) and Annona muricata (L.) (soursop) for their edible fruits. The importance of the A. muricata species in different applications is highlighted. Various parts of this plant are traditionally used to treat cancer, diabetes, hypertension, fever, gastrointestinal disorders, and parasitic infections, as well as the antimicrobial properties of the leaves used to treat viral infections (Pinto et al., 2017).
Among the main metabolites of this family, are acetogenins which have cytotoxic, antitumor, and insecticidal activity (Osorio et al., 2007); isoquinoline alkaloids, which have neurotoxic and antidepressant effects; and, to a lesser extent, phenolic compounds, cyclopeptides, vitamins, glycosides and volatile compounds (Pinto et al., 2017).
In Colombia, the main Annonaceae that are cultivated for its production are: A. muricata, A. squamosa and A. cherimola. Another minor species found in the wild is Rollinia mucosa ([Jacq.] Baillon). The greatest diversity of species is found in the Amazon (54%), Pacific (27.5%) and Andean (27%) regions. It 87% grow at altitudes of less than 500 m. The Annonaceae family is important from the nutritional point of view, around 4,626.4 ha planted belonging to the Annonaceae family, where 1,700 ha belong to crops of A. muricata, located mainly in the departments of Tolima, Valle del Cauca and the Eje Cafetero (León-Méndezet al., 2020).
Therefore, this research was carried out with the purpose of expanding the search for natural sources with antibacterial potential, through the bioprospecting study of Annonacea species cultivated in Colombia. The activity of extract from the seeds of A. muricata, A. cherimola, Annona glabra, Annona reticulata, R. mucosa and Annona montana was evaluated against the pathogens S. aureus, E. faecalis, B. subtilis, E. coli and P. aeruginosa, by the agar diffusion method. Additionally, a preliminary phytochemical study was made to establish to which type of compounds the activity found could be attributed.
MATERIALS AND METHODS
Plant material
The seeds were obtained from different Annonaceae ripe fruit species and were collected in different locations in Colombia as indicated in table 1. The taxonomic identification of A. montana and A. glabra took place in the herbarium at Universidad del Quindío with voucher number 38331 and 38332, respectively. R. mucosa was confirmed at the Herbarium of the Universidad de la Amazonia (HUAZ) with voucher number 1525. A. reticulata it was identified by Francisco Javier Roldan Palacio, curator of the Herbarium of the University of Antioquia (HUA). A. cherimola it was verified by the agronomist Hernán Giraldo of the Santa Rosa de Cabal University Corporation (UNISARC) and A. muricata it was verified by the agronomist Juan Felipe Romero.
Table 1. Names of the fruit species of the Annonaceae family cultivated in Colombia.
| Scientific name | Common name | Collection site | Latitude - Longitude |
|---|---|---|---|
| Annona muricata (L.) | Soursop | Pereira, Risaralda | 4°48´27.3´´ N 75°46´0.3´´ W |
| Annona montana (Macfad.) | Mountain soursop | Caicedonia, Valle del Cauca | 4°21´11.6´´ N 75°51´26´´ W |
| Annona glabra (L.) | Pond apple | Toro, Valle del Cauca | 4°41’11.77 N 76°2’42.05 W |
| Rollinia mucosa ([Jacq.] Baillon) | Wild sugar apple | Santo Domingo, Caqueta | 1°35´22´´ N 75°38´0.2´´ W |
| Annona cherimola (Miller) | Cherimoya | La bella, Risaralda | 4°45´55.9´´ N 75°38´17´´ W |
| Annona reticulata (L.) | Custard apple | Zarzal, Valle del Cauca | 4°23´30.2´´ N 76°04´37.3´´ W |
Obtaining the extracts
Seeds were washed with TEGO 51 (Quios, Bogota) and dried at 37ºC for 72 h accordance with that used in previous studies (Giraldo et al., 2020). Then, seeds were crushed in a mill MF 10 Basic (IKA, Campinas, Brazil). The powder obtained was carried out in an extraction process using the passive maceration method at room temperature. It was used absolute ethanol as solvent extraction with a solvent: sample ratio of 1:4 and regular agitation. The extract was filtered and concentrated by rotary evaporator until a viscous consistency and residual solvent was removed by nitrogen flow (Giraldo and Guerrero, 2018).
Antibacterial activity
The antibacterial activity of the Annonaceae extracts was tested against Gram-positive bacteria Staphylococcus aureus (Rosenbach) (ATCC 6538), Enterococcus faecalis ([Andrewes and Horder] Schleifer and Kilpper-Bölz) (ATCC 51299), and Bacillus subtilis ([Ehrenberg] Cohn) (ATCC 21556) and Gram-negative bacteria Escherichia coli ([Migula] Castellani and Chalmers) (ATCC 9637) and Pseudomonas aeruginosa ([J.Schröter] Migula) (ATCC 9027).
Agar diffusion method (zone inhibition assay). The determination of antibacterial activity was based according to the protocol provided in the Institute of Clinical and Laboratory Standards (CLSI, 2018) and with the methodology by Boorn et al. (2010).
Inoculum preparation. The inoculum was prepared from an actively growing culture plate (DifcoTM nutrient agar REF. 213000) for 24 h incubate at 37°C; with the aid of a bacteriological loop, colonies were taken and dissolved in distilled water, adjusting the inoculum to a turbidity of 0,5 MacFarland in a DensiCHEK™ Plus.
Inoculation of the culture medium. Agar plates of Mueller-Hinton agar (OXOID, CM0337) were inoculated using a swab from a suspension of each organism containing 1·106 CFU/mL by deep culture technique using Mueller-Hinton agar in 10 cm diameter Petri dishes was implemented, each microorganism was in a concentration at 0.2% v/v by exception from E. coli which was prepared at 0.5% v/v. A 3-mm diameter well was cut into the agar once it solidified.
Solutions preparation. The extracts crude was diluted in a small amount of dimethyl sulfoxide (DMSO). Commercial antibiotics were used as positive control. E. coli was tested at a concentration of 100 μg mL-1 amoxicillin (Genfar® 2007M-007513-R2); S. aureus and E. faecalis were tested at a concentration of 200 and 700 μg mL-1 ampicillin (Genfar® 2007M-07219-R2); P. aeruginosa and B. subtilis were tested at a concentration of 50 and 1000 μg mL-1 ciprofloxacin (Genfar® 2016M-001483-R2). Two controls were employed, a blank containing the same amount of dimethyl sulfoxide (DMSO) was used to dissolve the extracts and ethanol absolute was used a negative control.
Determination antibacterial activity of the extracts. 60 μL of each extract and the different control to be evaluated was aliquoted into each well. The dishes were incubated at 37°C for 24 h. After incubation, the diameter of the zone of inhibition was measured and the activity was determined by establishing the % inhibition by compared to the reference control. Each sample was tested a minimum of three times and means were calculated.
Phytochemical analysis of Annonaceae extracts
A phytochemical assay was performed, and the analysis of saponins and tannins, reducing sugars, total sugars, flavonoids, and glycosides (Elisha et al., 2017) was performed in tube tests. The analysis of isoquinoline alkaloids, phenols, terpenoids, lignans, sesquiterpene lactones, sterols, anthraquinones, and anthrones was conducted using thin layer chromatography (TLC), as well as the analysis of acetogenins and alkaloids (Giraldo and Guerrero, 2018).
Data analysis
The zone of inhibition (IZD) was measured in millimeters (mm) using the Image J program and the results were expressed as percentage inhibition (%). The inhibition halo of the controls was used as a reference parameter. All experiments were performed in triplicate and data are presented as mean values±standard deviation. The statistical differences between the treatments and the control were evaluated using the ANOVA test followed by Bonferroni (P>0.05).
RESULTS AND DISCUSSION
The average yield of seed ethanol extracts the different species of Annonaceae family are indicated in table 2. The range of ethanolic extraction by passive maceration are located between 14 a 40%.
Table 2. Yield of seed ethanol extracts the different species of Annonaceae family.
| Species | Seed mass (g) | Extract mass (g) | Yield (%) |
|---|---|---|---|
| A. muricata | 50.0817 | 7.3412 | 14.65 |
| A. montana | 31.5852 | 6.0830 | 19.26 |
| A. glabra | 18.4550 | 4.1816 | 22.65 |
| R. mucosa | 30.2240 | 6.5534 | 21.68 |
| A. cherimola | 30.0030 | 12.0234 | 40.07 |
| A. reticulata | 30.0047 | 8.4031 | 28.01 |
The antibacterial activity for six plant extracts against the five bacterial strains examined were assessed by the presence or absence of inhibition zones. The percentage inhibition of species is given in the figure 1. These results indicate that there is no homogenous antimicrobial activity behavior of the plant extracts. The inhibitory activity varied between 10 and 80%. The most sensitive bacterial strain was B. subtilis, in which the behavior was different against each extract, with significant differences of the extracts of A. glabra and A. reticulata that obtained inhibition percentages higher (Fig. 1E), in some cases, no colony growth was observed in the medium, and in others, translucent dotted halos were observed (Fig. 2). On the other hand, P. aeruginosa and E. coli were the most resistant to the extracts analyzed, however, percentage inhibition of A. glabra of 40% was observed in P. aeruginosa (Fig. 1A).

Figure 1. Percentage of inhibition of extract Annonacea against: A) P. aeruginosa, B) E. faecalis, C) S. aureus, D) E. coli, E) B. subtilis. Different letters indicate significant differences between extracts according to Bonferroni (P≤0.05), n=3±SE.
The A. montana extract presented activity against the five bacterial strains, where the most important results were against S. aureus (34.87±15.37%) and E. faecalis (30.61±1.04%). The A. cherimola extract exhibited the highest percentage of inhibition against E. faecalis (79.86±3.81%), with significant differences with the results obtained by the extracts of the other two species (A. montana with 30.60 ±1.04% and A. glabra with 27.75±6.69%) (Fig. 1B).
The extracts of the species A. montana, A. reticulata and A. glabra were the only ones that showed activity against S. aureus, without presenting significant differences between the percentages of inhibition. On the other hand, the extract of A. muricata was the only one that did not present any activity against the microorganisms evaluated in this study.
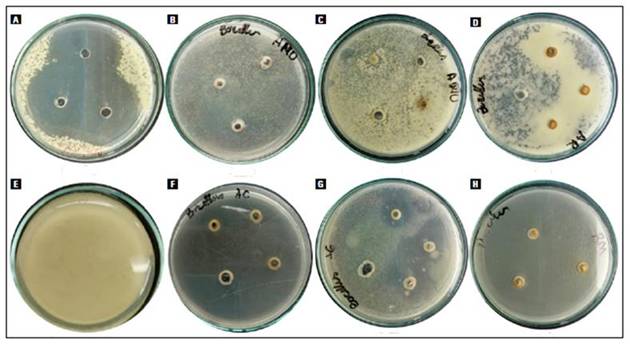
Figure 2. Inhibition of the growth of B. subtilis against (A) Antibiotic (B) A. montana (C) A. muricata (D) A. reticulata (E) Growth control (F) A. cherimola (G) A. glabra (H) R. mucosa.
The chemical analysis of the Annonaceae crude extracts showed the presence of isoquinoline alkaloids, anthrones, glycosides, and acetogenins for the six species tested. Only A. montana and A. glabra were positive for sesquiterpene lactones but negative for sterols. Sugars were present in A. muricata, A. glabra and A. montana, and saponins were detected only in A. cherimoya, A. reticulata and A. montana. This allowed the detection of the presence of different phytochemical constituents (Tab. 3) that could be responsible for the antibacterial activity.
Table 3. Phytochemical analysis of extracts from Annonaceae species.
| Metabolites | A. muricata | R. mucosa | A. cherimola | A. glabra | A. reticulata | A. montana |
|---|---|---|---|---|---|---|
| Isoquinoline alkaloids | + | + | + | + | + | + |
| Phenols and flavonoids | - | - | - | - | - | - |
| Terpenoids and lignans | + | + | - | + | + | + |
| Sesquiterpene lactones | - | - | - | + | - | + |
| Sterols | + | + | + | - | + | - |
| Terpenoids | - | - | + | - | - | - |
| Anthraquinones | - | - | - | - | - | - |
| Anthrones | + | + | + | + | + | + |
| Coumarins | - | - | - | - | - | - |
| Acetogenins | + | + | + | + | + | + |
| Alkaloids | + | + | + | + | + | + |
| Reducing sugars | + | - | - | - | - | - |
| Total sugars | + | - | - | + | - | + |
| Flavonoids | - | - | - | - | - | - |
| Tannins | - | - | - | - | - | - |
| Glycosides | + | + | + | + | + | + |
| Saponins | - | - | + | - | + | + |
In general, it was observed that the Annonaceae extracts evaluated had higher activity against Gram-positive bacterial strains. This may be because Gram-negative strains are more resistant due to the structure of the cell wall, which acts as a permeability barrier, reducing the uptake of compounds into the cell (Elisha et al., 2017). One of the most important results found in this study is the activity of A. cherimola against E. faecalis (79.86±3.81 %) and that of R. mucosa against E. coli (58.95±13.63). It is important to highlight the result obtained by A. reticulata against B. subtilis (Fig. 2), where a halo of greater growth of the microorganism was observed around the well, a phenomenon that should be studied in greater depth. A. cherimola is a fruit known for its high cytotoxic potential due to the presence of acetogenins, compounds that are characterized by the activation of apoptotic pathways, which leads to cell death (Haykal et al., 2019). This allows establishing these compounds as responsible for the activity presented by these fruits, as has been reported in other studies, the acetogenesis of this fruit presents an important activity against gram positive bacteria (Aguilar-Villalva et al.,2021; Perrone et al., 2022).
R. mucosa is considered an exotic Amazonian fruit. Previous reports have indicated that the hexane extracts of the seeds, pulp and stems were evaluated against five microorganisms, where the highest result corresponded to stem extracts against Candida albicans with a percentage of inhibition of 39.14% (Fernández et al., 2020), being the result obtained in this study promising for this fruit species.
On the other hand, the extracts of the A. reticulata leaf have shown inhibitory activity against P. aeruginosa, identifying compounds of the alkaloid, flavonoid, phenol, steroid and tri-terpene type (Harahap et al., 2022), also identify in this study. Terpenes are attributed to the disruption capacity of the bacterial cell membrane (Saleem et al., 2003). Saponins have been associated with the ability to produce pores in the cell membrane, forming complexes with cholesterol, altering cell morphology, especially in Gram-positive bacteria (Arabski et al., 2009). These two types of compounds could explain the result observed with this extract compared to B. subtilis.
It is important to comment that A. muricata has been the most studied species, where the polar extracts of the leaves with the presence mainly of isoquinoline alkaloids act against multiresistant bacteria, especially E. faecalis, E. coli and P. aeruginosa with promising results (Tojola et al., 2019; Iyanda-Joel et al., 2019; Aguilar-Hernández et al., 2023). Although, in this study, A. muricata did not present bactericidal activity, this can be attributed to the fact that the extract evaluated was from the seed, with other types of metabolites present. Another example of this is A. glabra where stem bark extracts, flavonoid-like compounds with antimicrobial properties against P. aeruginosa have been identified (Galvão et al., 2016) and this allows highlighting the importance of the type of extract obtained and the type of functional groups identified in the preliminary phytochemical analysis.
Additionally, it is observed that the response of the microorganisms against the extracts was diverse, especially with E. coli and B. subtilis. The growth of the microorganisms presented visual changes or in some cases it was inhibited, indicating that the antimicrobial activity of the extracts could be associated with the effect on the target site on the cell walls as well as with the type of interacting metabolites. The mechanisms include the inhibition of the cell wall, the blocking resistance mechanisms, the inhibition of protein synthesis, and metabolism (Calvo and Martínez-Martínez, 2009).
The results obtained in the phytochemical assays suggest that the Annonaceae extracts may have different antimicrobial activities because differences in the composition of the secondary metabolites were found. According to previous studies, the antimicrobial activity depends on the type of metabolite present (Maillard, 2002), which can exhibit different modes of action or be associated with microorganisms that more effectively overcome the effect of a compound or adapt to it (Berić et al., 2018; Çördük et al., 2017; Gavrilović et al., 2016; Ji et al., 2016; Maya et al., 2021).
All the evaluated extracts tested positive for alkaloids and glycosides; alkaloids are good bactericidal agents and exert their activity on the membrane. These compounds disrupt the formation of the bacterial Z-ring and inhibit bacterial cytokinesis, which ultimately leads to inhibition of cell reproduction (Barbieri et al., 2017; Nájera-Arce et al., 2018). Additionally, different mechanisms of action have been proposed for glycosides, with indications that they act mainly in the membrane to alter cellular permeability and as chelating agents and inhibit the production of toxins and microbial growth (Mullins, 1990; Rabea et al., 2003). Sterol-derived compounds have been reported as bacteriostatic agents because they have strong inhibitory effects (Hovenkamp et al., 2008; Bhardwaj et al., 2014).
Although not all extracts showed activity against the different strains evaluated in this study, it has been reported that extracts from species of the Annonaceae family can modulate drug resistance (Barboza et al., 2015). These results indicate that the extracts have good potential for therapeutic uses against some pathogens.
CONCLUSION
According to the results, ethanolic extracts obtained from the seeds of the main Colombian species of Annonaceae showed antimicrobial properties against the microorganisms S. aureus, E. faecalis, B. subtilis, E. coli and P. aeruginosa; it suggests that different extracts contain compounds with antibacterial properties, and it is according to their phytochemical differences. These results contribute to the bioprospecting of this family since previous studies have been carried out mainly with extracts obtained from the leaves.
Owing to the potential antibacterial actions of Annonaceae fruit, it is possible to continue for further studies aimed identifying the single active principles and evaluating possible synergism of antimicrobial activity among these extracts. The applications could an accessible and safe alternative to synthetic antimicrobial drugs.





![Variability, correlation, and path analysis in erect and prostrate cultivars of cowpea (Vigna unguiculata [L.] Walp.)](/img/en/next.gif)









