INTRODUCTION
Household detergents are a mixture of 20 or more ingredients, among which stand out surfactants, adjuvants, bleaching agents, foaming agents, softeners and sometimes enzymes, anti-depositing agents, optical brighteners and perfumes. Surfactants are the main ingredients and among them sodium dodecylbenzene sulfonate stands out. Adjuvants are the second most important component of these formulations after sodium tripolyphosphate (Na5P3O10) but are recognized as a dangerous environmental pollutant causing eutrophication (Showell, 2016).
The increasing pollution derived from the use of synthetic detergents has been reflected in numerous bodies of water and soils in different regions of the world. Mexico has not been exempt from this and evidence of these problems is the Rio San Pedro in Aguascalientes, Chetumal Bay in Quintana Roo (Uc-Peraza and Delgado-Blas, 2015; Fernández-Ronquillo et al., 2016). It has also been observed that the continuous discharge of greywater contaminated with detergents has affected the physical and chemical properties of the soil in the backyards of rural houses in Hunucmá, Yucatán (Estrada-Medina et al., 2018).
The arrival of the COVID-19 pandemic had a negative impact on these issues, as it led to a sudden increase in the use of detergents in all households in a short period of time (Mohamed et al., 2022). This phenomenon resulted in a higher generation of liquid soap waste worldwide (Rafieepoor et al., 2021) and particularly in Mexico, according to El Economista magazine. During the first four months of 2020, the consumption of laundry soaps and detergents increased by 47 and 33%, respectively.
In several countries, the use of domestic greywater contaminated with detergents for agricultural irrigation has become widespread, potentially posing a significant risk to soil health and plant growth. Consequently, there has been a growing focus on assessing the impact of detergents on crop development (Lal, 2003; Ehilen et al., 2017)
Some studies have shown that detergents applied in controlled and relatively low concentrations (1 and 2 mg L-1) can have positive effects on plants (Kroontje et al., 1973; Mohammad and Moheman, 2012). However, at higher concentrations (10, 100 and 500 mg L-1), detergents decrease germination, growth, photosynthetic activity and chlorophyll content and increase accumulation of sodium (Na), potassium (K), calcium (Ca) and soluble proteins in crops such as tomato (Solanum lycopersicum) (Ehilen et al., 2017), mung bean (Vigna radiata) (Lal, 2003), maize (Zea mays) (Uzma et al., 2018), lettuce (Lactuca sativa) and okra (Abelmoschus esculentus) (Sawadogo et al., 2014).
In the rural communities of Yucatán, there is no drainage system, and wastewater from laundry, dishwashing, and household cleaning has been discharged onto backyard soils for decades (Estrada-Medina et al., 2018). Due to the increased prevalence of these practices in many regions of the world, along with the need to establish appropriate measures for greywater reuse in agriculture, it is essential to understand and study the effects of detergents on soils and plants.
The species Capsicum chinense Jacq., commonly known as habanero pepper, is one of the emblematic commercial crops of the Yucatán Peninsula, and in rural areas of the state, small-scale farmers grow habanero peppers for family consumption and sell them in small quantities locally (Flores-López and Sánchez-Osorio, 2020). Furthermore, due to its ease of cultivation under in vitro and greenhouse conditions, it has been used as a model for studying its response to various biotic and abiotic environmental stress conditions (Borges-Gómez et al., 2014)
Information regarding soil-plant interaction and abiotic stress caused by detergent contamination is highly limited and of vital importance for designing proper wastewater management. This study represents a preliminary investigation in this field and aimed to assess the effects of three household detergents on the germination and initial growth of habanero pepper plants.
MATERIALS AND METHODS
Characteristics of used detergents
For this study, three detergents that differ in their P contents were selected that, according to surveys in 2016, were the most used in cleaning activities in the municipality of Hunucma (Yucatan) (Estrada-Medina et al., 2018). Table 1 shows information on the composition of the three detergents supplied by the manufacturer.
Table 1. Chemical composition reported by manufacturers for detergents D0P (phosphorus-free detergent), D1P (phosphorus detergent), and D2P (detergent with twice the phosphorus of D1P), which were used to evaluate their effects on the germination and growth of Capsicum chinense (Jacq.).
| Det | Reported chemical composition | Manufacturer |
|---|---|---|
| D0P | LAS, Na2CO3, Na2SiO3, C6H8O7, C13-C15 | Procter & Gamble, Spain |
| D1P | LAS, Na2CO3, Na2SiO3, Na2SO4, Na5P3O10 | Fábrica de Jabón La Corona, Mexico |
| D2P | LAS, Na2SiO3, Na5P3O10, CMC, proteolytic enzyme and additives | Fábrica de Jabón La Corona, Mexico |
Det: detergent; LAS, linear alkylbenzene sulfonate; Na2CO3, sodium carbonate; Na2SiO3, sodium silicate; C6H8O7, citric acid; C13-C15: dispersing polymer (Parenth C13-C15); Na2SO4, sodium sulfate; Na5P3O10, sodium tripolyphosphate; CMC: antidepositing agent, Additives: bleaches and perfumes.
In all three detergents, the contents of Na and Ca were determined by extraction in ammonium acetate and subsequent determination by flame photometry (4616-BWB, XP) (Sparks, 1996). Also, the P content was determined by extraction in sodium bicarbonate (NaHC03 0.5 N) and determination by UV-Vis spectrophotometry (Thermo spectronic, genesis series 10) (Sparks, 1996). The analyses were carried out in the Soil, Plant and Water Analysis Laboratory of the Biological and Agricultural Sciences Campus of the Universidad Autónoma de Yucatán.
Effects of detergents on seed germination and seedling vigor
The first experiment was conducted at the Soil, Plant and Water Analysis Laboratory of the Biological and Agricultural Sciences Campus of the Universidad Autónoma de Yucatán in December 2020. For the study, seeds of habanero pepper (Capsicum chinense Jacq.), Jaguar variety, from the company Seminis were used. Initially, these seeds were disinfected according to the protocol used by Bojórquez-Quintal et al. (2014). The disinfected seeds were placed in Petri dishes on filter paper moistened with 10 mL of the solutions corresponding to each treatment. The experiment consisted of 13 treatments that included a control (0 mg L-1- deionized water) and four concentrations (50, 500, 1,000 and 2,000 mg L-1) of three detergents (D0P, D1P and D2P). Five petri dishes were used per treatment with 30 seeds each. A completely randomized experimental design was followed.
Petri dishes were kept in a growth chamber model CA550 (Novatech, Mexico) under dark conditions and at a temperature of 25±2°C (Bojórquez-Quintal et al., 2014). After 10 d, 10 mL of the corresponding solution was added to each Petri dish with the aim of maintaining the necessary humidity for the germination process. The daily count of germinated seeds was carried out from day five, when the emergence of the radicles began, until day 20 after imbibition. Day 12 was considered the last day of germinated seed counting, as no new radicle emergences were observed in subsequent days.
After 20 d, total fresh weight (mg) was measured in 20 seedlings per petri dish using an analytical balance. In addition, the germination percentage (% germination) and the vigor index (VI) were calculated according to the formulas of Shumaila and Sami, (2019).
Effects of detergents on seedling growth in hydroponic conditions
The second experiment was conducted at the Biochemistry Unit of the Yucatan Scientific Research Center in December 2021. In this experiment, the detergents D0P and D2P were chosen because they exhibited the most prominent inhibitory effects in the previous experiment. Likewise, concentrations of 500 and 2,000 mg L-1 were selected as they demonstrated contrasting effects.
The habanero pepper seeds of the Jaguar variety were disinfected as described above, placed in petri dishes and after protrusion of the radicle (4 to 5 d), and then transferred to plastic containers (450 mL) containing vermiculite with 90 mL of Hoagland's nutrient solution added (Hoagland and Arnon, 1950). The containers were placed in laboratory conditions, under a photoperiod of 16 h light and 8 h darkness, at 25+2°C and light intensity of approximately 120 μmol m-2 s-1.
After 5 d of growth, when the cotyledons emerged, the vermiculite was carefully removed and the seedlings were placed in hydroponic conditions, with Hoagland solution. After another 5 d in hydroponics, the nutrient solution was prepared again and the detergent treatments were applied. There were five treatments: a control (0 mg L-1) and two concentrations (500 and 2,000 mg L-1) of two detergents (D0P and D2P). Three hydroponics containers were used per treatment with 12 seedlings each. A completely randomized experimental design was followed.
Root growth was recorded by capturing images on days zero (day of application of treatments), three and seven. Images with a resolution of 300 dpi were captured using an Epson Perfection 3490 Photo scanner. The number of lateral roots, the total length of the root system, and the leaf and root area were determined through Regent Instruments' Rootedge software. In addition, the foliar and root fresh weight was determined by means of an analytical balance and the foliar fresh weight/root fresh weight and foliar fresh weight/leaf area ratios were calculated. Evaluations were conducted on 6 plants per treatment.
A bivariate analysis (two-way ANOVA) was used to process data from both experiments. In this analysis, detergent types were considered as factor 1 and concentrations as factor 2. To analyze the differences between the treatments, Duncan's Multiple Range Comparison test was applied with a significance level of P≤0.05. All statistical analyses were performed using SPSS Statistics software, v. 22 for Windows.
RESULTS AND DISCUSSION
Characteristics of used detergents
Table 2 shows the contents of P, Na, and Ca in each detergent. The difference between the three detergents was observed in the P contents, as D0P does not contain P, and D2P contains twice as much P as D1P; for this reason, they were identified with the mentioned names. It can also be noted that no differences were found in the Na and Ca contents. It is likely that this difference in P contents, along with the differences in composition, may lead to different effects on plant germination and growth.
Table 2. P, Na and Ca content in detergents D0P, D1P and D2P (mg kg-1).
| Detergents | P (mg kg-1) | Na (mg kg-1) | Ca (mg kg-1) |
|---|---|---|---|
| D0P | 0 c | 32,140 a | 17,949 a |
| D1P | 7,162 b | 32,176 a | 18,287 a |
| D2P | 14,256 a | 30,813 a | 17,584 a |
| SE | 636 | 855 | 919 |
SE: standard error of the mean; D0P: detergent 1 without phosphorus, D1P: detergent 2 with phosphorus; D2P: detergent 3 with twice the phosphorus than D1P. Capital letters compare different detergents, according to Duncan (P≤0.05) (n=3). Means with equal letters show no significant differences.
Effects of detergents on seed germination and seedling vigor
Germination percentages close to 90% were recorded in all treatments (Tab. 3), which is within the expected range for the Jaguar variety as reported by the seed producer. Increase in detergent concentrations did not affect the percentage of final germination (Tab. 3). However, in other crops such as tomato, sunflower (Helianthus annuus), quintonil, lentils and different varieties of beans, reductions in germination percentage have been reported due to exposure to household detergents at concentrations similar to those used in this study (Lal, 2003; Heidari, 2013; Ehilen et al., 2017; Ikhajiagbe et al., 2020; Cai and Ostroumov, 2022). The absence of germination has been observed in mung bean and quintonil at concentrations of 1,500 and 2,500 mg L-1, respectively (Lal, 2003; Ehilen et al., 2017).
Table 3. Germination percentage of habanero pepper seeds (Capsicum chinense Jacq.) var. Jaguar, exposed to different concentrations of detergents D0P, D1P and D2P during 20 days of treatment.
| Detergent concentration (mg L-1) | % germination D0P | % germination D1P | % germination D2P |
|---|---|---|---|
| 0 | 94 | 94 | 94 |
| 50 | 97 | 96 | 97 |
| 500 | 92 | 93 | 92 |
| 1,000 | 98 | 93 | 95 |
| 2,000 | 94 | 94 | 93 |
| SE | 2 | 2 | 2 |
SE: standard error of the mean; D0P: detergent 1 without phosphorus, D1P: detergent 2 with phosphorus; D2P: detergent 3 with twice the phosphorus than D1P. The type of detergent, concentrations and interaction detergent x concentration were not significant (P>0.05) (n=5).
It was observed that detergents had an impact on the germination rate, which was dependent on both the type and concentration of detergent (Fig. 1). In the case of the D0P detergent at a concentration of 2,000 mg L-1, a delay was observed in the start of the germination process, with a lower percentage of germination recorded on days five, six and seven compared to the control. However, by day eight, germination was accelerated and values similar to the control were reached, which were maintained until the end of the evaluation (Fig. 1A).
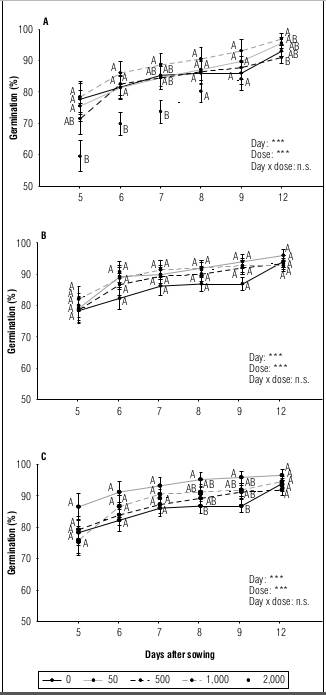
Figure 1. Germination dynamics for habanero pepper (Capsicum chinense Jacq.) var. Jaguar exposed to different concentrations (0; 50; 500; 1,000 and 2,000 mg L -1 ) of detergents D0P (A), D1P (B) and D2P (C). The data are shown as mean value + standard error of the mean (n=5). Boxes: two-factor ANOVA (n.s.: not significant P>0.05; ***: P<0.001). The letters compare different concentrations on the same day, according to Duncan (P≤0.05).
During the germination process, seeds not exposed to detergent treatments as well as those exposed to D0P detergent (Fig. 1A) in concentrations of 1,000 and 2,000 mg L-1 reached their maximum germination percentage 12 d after sowing. However, seeds exposed to D1P and D2P at concentrations of 1,000 and 2,000 mg L-1 reached their maximum percentage on day six (Fig. 1B and 1C), indicating earlier germination in these treatments. In addition, seeds exposed to 50 mg L-1 treatment of the D2P detergent showed a higher percentage of germination compared to the control on days eight and nine (Fig. 1C). These results show that the presence of P accelerates the speed of germination.
One of the most important aspects of germinative metabolism at the beginning of imbibition is the production of ATP. For this, it is necessary to trigger hydrolysis reactions in the phosphorylated reserves of the embryo. With the plant growing, most phosphate ions are transported from the aleurone cells, where the hydrolysis of the phytin and phytate reserves occurs, to the organs of active growth (Azcón-Bieto and Talón, 2013). It is likely that phosphate detergents accelerate the germination process because phosphate ions can quickly reach the embryo through imbibition water and initiate metabolic processes without prior hydrolysis reactions.
The lower germination percentage in the 2,000 mg L-1 treatment with D0P at 5 d after sowing suggests that surfactants or other components of this phosphorus-free detergent may delay the onset of germination. On the other hand, this inhibitory effect may not have been evident in the D1P and D2P treatments due to the compensatory effect induced by the presence of phosphorus, which could mask the toxicity of the surfactants during these early exposure days.
Regarding the fresh weight and vigor index of germinated seedlings it was observed that detergents D0P and D2P caused a biphasic effect; stimulatory in the concentration of 500 mg L-1 and inhibitory in the concentration of 2,000 mg L-1. The same tendency was found for D1P, but without statistical differences from the control (Fig. 2).
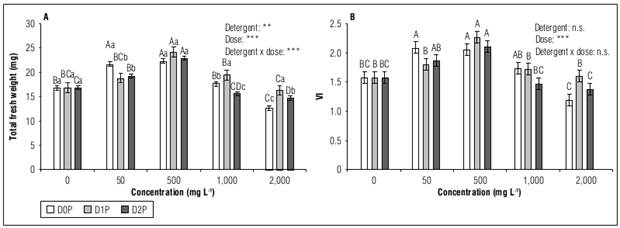
Figure 2. Total fresh weight (A) and seedling vigor index-VI (B) of habanero pepper (Capsicum chinense Jacq.) var. Jaguar after 20 days exposureto different concentrations (0; 50; 500; 1,000 and 2,000 mg L -1 ) of D0P, D1P and D2P detergents. The data are shown as mean value + standard error of the mean (n=100 for A and n=5 for B). Inset: two-factor ANOVA (n.s.: not significant P>0.05; **: P<0.01; ***: P<0.001). Uppercase letters compare different concentrations in the same detergent and lowercase letters compare different detergents in the same concentration, according to Duncan (P≤0.05).
The magnitude of effects on fresh weight and response at some concentrations were not equal for each detergent. Unlike D1P and D2P, stimulatory effects of D0P were observed from 50 mg L-1. In addition, at 2,000 mg L-1 the three detergents showed statistical differences: D0P reduced weight more than D2P, which in turn reduced weight more than D1P (Fig. 2A).
Most studies conducted on other crops of similar age have found no stimulating effects of detergents on growth (Lal, 2003; Heidari, 2012, 2013; Issayeva et al., 2015, Uzma et al., 2018). In contrast, the growth-stimulating concentrations in the present study led to reductions in other crops, such as lettuce and mung beans (Lal, 2003; Sawadogo et al., 2014) and showed no significant effects in other less sensitive ones, such as maize, sunflower and black beans (Heidari, 2012, 2013; Issayeva et al., 2015; Uzma et al., 2018).
According to the available literature, only Kroontje et al. (1973) found that exposure to a household detergent at concentrations close to 800 mg L-1 caused increases in the growth of corn seedlings after 20 d of germination. Also, other studies in higher phenological stages show that low concentrations of detergents can have stimulating effects on growth and yield, both in the case of irrigation with wastewater (Rodda-Rodda et al., 2011) and in exposure to solutions of household detergents (Kroontje et al., 1973) and surfactants (Mohammad and Moheman, 2012). The authors of these studies associated increases in growth with increased availability of nutrients such as P (Kroontje et al., 1973; Rodda-Rodda et al., 2011). However, in the present study, P-free detergent also stimulated growth, suggesting that there are other non-studied detergent components contributing to these effect.
The stimulating effect of detergents could be associated with the presence of surfactants, as these molecules can serve as a carbon source and reduce the surface tension of membranes, which facilitates nutrient absorption (Mohammad and Moheman, 2012). Additionally, surfactants can interact with membrane lipids and proteins, increasing their extensibility and promoting cell elongation. Furthermore, the presence of calcium ions provided by the three detergents may enhance the activity of gibberellins (GAs), which can induce the synthesis and secretion of α-amylase to initiate the degradation of substances mobilized in the reserve organs and transport them to the growth zones of the radicle (Azcón-Bieto and Talón, 2013).
The toxic effect observed at high concentrations of 1,000 and 2,000 mg L-1 may be the result of the salt content, and the toxicity of the surfactant, or a combination of both effects. The previous studies show that surfactants facilitate the absorption of salts into the leaf tissues, which triggers a synergistic action of dual toxic effects caused by salts and surfactants (Toscano et al., 2022).
No differences in Na contents were found between the three detergents (Tab. 2). Therefore, it considers that this element is not related to the difference in effects between detergents at the highest concentrations. It is possible that the greater inhibitory effect caused by D0P at 2,000 mg L-1 is due to the delay observed in the start of germination, which limited the growth time of seedlings. In addition, the stimulating effects at low concentration (500 mg L-1) of D1P may be due to the presence of sulfur in the form of Na2SO4. The sulfur composes amino acids, such as cysteine and methionine, as well as various coenzymes such as thiamine, biotin and coenzyme A, which is a key compound in the activation of organic acids (Azcón-Bieto and Talón, 2013). Therefore, it can promotethe processes of synthesis and degradation of fatty acids and in cellular respiration.
In addition to the reduction in fresh weight, it was also observed that seedlings germinated under the elevated conditions of 1,000 and 2,000 mg L-1 showed dark coloration, which was more significant at the highest concentration. These changes are considered to be the result of oxidative processes, since the surfactants interact with various proteins and lipids of the cell membrane, which alters the normal structure of the lipid bilayer and affects the physiological and biochemical processes of the cells. The outcome of this interaction depends on concentration. The high concentrations of surfactants solubilize membranes and cause cell lysis, while the low concentrations can induce a negatively charged surface (Parsi, 2014).
Effects of detergents on seedling growth under hydroponic conditions
In the second experiment, it was observed that at day 3 of treatment, day 13 after germination, both detergents caused inhibitory effects on growth, which were manifested by the following factors, such as the leaf and root area, fresh weight, the total length of root, and the number of lateral roots (Tab. 4). Considerable reductions from the first concentration were observed, in which most indicators ranged by 50%, and with no major effects in the second concentration.
Table 4. Leaf area and root area, leaf fresh weight and root fresh weight, root length and number of lateral roots of habanero pepper (Capsicum chinense Jacq.) seedlings var. Jaguars exposed three and seven days to different concentrations of D0P and D2P detergents.
| Treatment | Leaf area (cm2) | Fresh foliar weight (mg) | Root area (cm2) | Root fresh weight (mg) | Root system length (cm) | Number of lateral roots |
|---|---|---|---|---|---|---|
| Day three of exposure | ||||||
| 0 | 2.9 A | 79.9 A | 2.3 A | 22.9 A | 25.8 A | 9.8 A |
| D0P 500 | 1.3 B | 45.2 B | 1.2 B | 9.5 C | 11.4 B | 3.2 B |
| D0P 2000 | 1.4 B | 44.2 B | 1.5 B | 13.9 B | 13.1 B | 4.5 B |
| SE | 0.1 | 4 | 0.1 | 1 | 1 | 0.7 |
| D2P 500 | 1.3 B | 48.5 B | 1.5 B | 11.5 B | 14 B | 6.0 B |
| D2P 2000 | 1.4 B | 44.0 B | 1.3 B | 12 B | 12.7 B | 5.3 B |
| SE | 0.2 | 4 | 0.1 | 2 | 1 | 0.7 |
| Day seven of exposure | ||||||
| 0 | 7 A | 154 A | 6 A | 61 A | 64 A | 24 A |
| D0P 500 | 1 B | 43 B | 1 B | 9 B | 8 B | 4 B |
| D0P 2000 | 1 B | 16 C | 1 B | 9 B | 12 B | 3 B |
| SE | 0.2 | 7 | 0.3 | 4 | 3 | 0.7 |
| D2P 500 | 2 B | 51 B | 1 B | 14 B | 11 B | 3 B |
| D2P 2000 | 1 B | 17 C | 1 B | 9 B | 10 B | 5 B |
| SE | 0.3 | 7 | 0.3 | 4 | 3 | 0.8 |
SE: standard error of the mean. The type of detergent and the interaction between detergent x concentration were not significant (P>0.05) (n=6). Capital letters compare different concentrations in the same detergent, according to Duncan (P<0.05). Means with equal letters show no significant differences.
Other studies in different species, such as quintonil, tomato (Ehilen et al., 2017), mung bean (Lal, 2003), corn (Heidari, 2012) and sunflower (Heidari, 2013), also found that exposure to certain concentrations of detergent inhibit seedling growth. The difference with these investigations is that the experiments were carried out in soil and, therefore, due to its buffering power, the roots are less exposed to the different toxic elements than if they are developed in hydroponics. This may be one of the reasons why the effects on growth are less pronounced and are observed at higher concentrations (2,000 mg L-1) (Lal, 2003; Heidari, 2012, 2013; Ehilen et al., 2017) than in the present study. However, in another study in hydroponics in aquatic plants (Azolla filiculoides and Lemna minor) exposed to the anionic surfactant sodium dodecyl sulfate (50 and 100 mg L-1), reductions in growth were also observed that were less pronounced than those of the present study (Forni et al., 2008).
At day seven of exposure to detergents and day 17 after germination, the negative effects in most indicators with respect to the control were higher than those at day three of exposure and ranged around 70% for both detergent concentrations (Tab. 4). Only in the fresh weight of the air organs did the concentration of 2,000 mg L-1 significantly increased the inhibitory effect with respect to the concentration of 500 mg L-1.
At day seven of exposure to detergents, the ratio between leaf and root fresh weight increased at 500 mg L-1 of two detergents (D0P and D2P) and remained near to the control at 2,000 mg L-1 (Fig. 3B). This phenomenon was again observed after three days of treatment in the D0P detergent (Fig. 3A). This indicates that the root was reduced more than the aerial part in the first concentration, while the reduction of both organs was similar to each other in the second concentration.
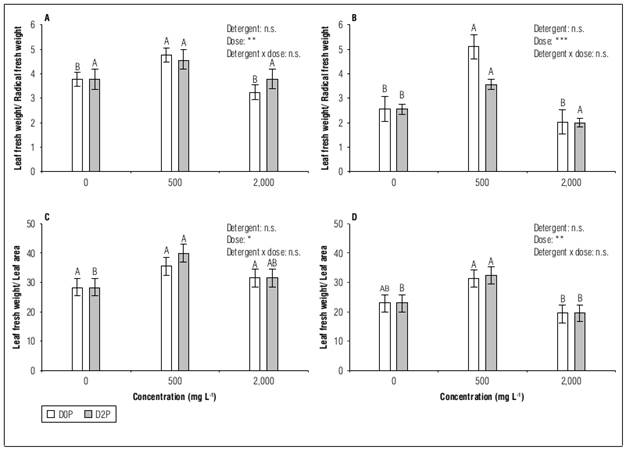
Figure 3. Ratio foliar fresh weight/root fresh weight of habanero pepper seedlings (Capsicum chinense Jacq.) var. Jaguar exposed three (A) and seven (B) days to different concentrations of D0P and D2P detergents. Foliar fresh weight/leaf area ratio after three (C) and seven (D) days of exposure. The data are shown as mean + standard error of the mean (n=6). Inset: two-factor ANOVA (n.s.: not significant P>0.05; **: P<0.01; ***: P<0.001). The letters compare the different concentrations of the same detergent, according to Duncan (P< 0.05).
The above results correspond to those obtained for the ratio between fresh weight and leaf area (Fig. 3C and D), since an increase in this indicator was also observed in the first concentration, which indicates that the leaf became thicker (greater specific weight of the leaf) under these conditions. This behavior has previously been documented in 20-d-old maize plants when exposed to a concentration of 20,000 mg L-1 of a P-free household detergent (Heidari, 2012). This thickening of the leaf may be a consequence of a decrease in cell size and an increase in the concentration of intracellular solutes due to water stress (Heidari, 2012).
In addition to the effects on growth, visual damage was also manifested in the development and coloration of leaves and roots. From the third day of treatment, seedlings exposed to all detergent treatments did not develop true leaves and showed darkening at the root apex. Similarly, seedlings exposed to 2,000 mg∙L-1 showed dark coloration in the veins and petiole of the cotyledons (data not shown).
On day 7 of treatment, differences were also observed between the appearances of seedlings of both concentrations; seedlings exposed to 2,000 mg L-1 showed greater darkening in the veins and petiole of the leaves than those exposed to 500 mg L-1 (Fig. 4). These symptoms do not correspond to the burns and necrosis on the edges of the leaves that are commonly observed when there is salt stress (Azcón-Bieto and Talón, 2013). Therefore, this darkening may be the product of possible cell death caused by the direct effect of surfactants transported through vascular tissues.

Figure 4. Seedlings of habanero pepper (Capsicum chinense Jacq.) var. Jaguar with 17 days of sprouts and 7 days of exposure to detergents. A: Control; B: Treatment with 500 mg L -1 of D0P; C: Treatment with 2,000 mg L -1 of D0P.
The 500 mg L-1 concentration of D0P and D2P detergents stimulated the vigor of germinated seedlings in experiment 1; however, it proved toxic when applied to 10-day-old seedlings under hydroponic conditions in experiment 2. This may be because older seedlings have more developed roots and have a greater need for nutrients; therefore, they absorb more of the toxic elements present in the medium.
This study does not only contribute basic knowledge to science, but supplement evidence of the negative impact on cropstriggered by detergents. It constitutes a preliminary study in the early stages of the life of the habanero pepper and identifies concentrations that begin to be harmful to plants, as well as the differences between the types of detergents evaluated.
The results showed that the dose of 500 mg L-1 is beneficial in germination and toxic at the beginning of the vegetative stage and that the dose of 2,000 mg L-1 is toxic at both times. Therefore, it is not advisable to use wastewater with detergent concentrations close to 500 mg L-1 for the irrigation of habanero peppers in the initial stage of growth. It was also observed that the presence or absence of phosphorus in the formulations of detergents did not have a significant impact. However, the presence of Na2SO4 in D1P detergent appeared to reduce the toxic effect compared to the other detergents.
CONCLUSIONS
The detergents did not affect the final percentage of germinated seeds, but they did modify the germination rate. The concentration of 2,000 mg L-1 of D0P delayed the onset of germination, while the concentration of 50 mg L-1 of D2P increased the number of germinated seeds on days eight and nine. All three detergents (D0P, D1P, and D2P) had a biphasic effect on the total fresh weight and vigor index of the seedlings: stimulatory at 500 mg L-1 and inhibitory at 2,000 mg L-1. The difference between them was that only D0P showed stimulatory effects at the 50 mg L-1 dose, and at 2,000 mg L-1, D0P reduced the weight to a greater extent than D2P, and in turn, D2P reduced it more than D1P. Both types of detergents (D0P and D2P), whether with or without P, are considered toxic when present in hydroponics at concentrations of 500 mg L-1, as they reduce root and leaf growth by approximately 50% and cause damage that affects most of the leaf tissue, within just three days of exposure.















