INTRODUCTION
Zinc is one of the essential elements for plant development, since it contributes to the stability of plasmalemma, the synthesis of cytochromes and chlorophylls, participates in photosynthetic processes, synthesis of lipids, carbohydrates, proteins and nucleic acids, determines the expression of some genes and regulates the activity of hormones, improves water ratios, the uptake of other nutrients and the accumulation of osmolytes (Hassan et al., 2022; Nekoukhou et al., 2022; Cakmak et al., 2023). Additionally, it is the only element that can be found in all six classes of enzymes, such as oxidoreductases, hydrolases, transferases, isomerases, lyases and ligases (Hassan et al., 2022; Nekoukhou et al., 2022). However, Zn at levels harmful to plants is found in some soils and in water sources as a result of weathering of rocks, forest fires, volcanic activity, recurrent application of phosphate fertilizers and the release of industrial effluents (Kaur and Garg, 2021; Hussain et al., 2022; Fischer and Fischer-García, 2023).
Furthermore, the continuous and excessive application of pesticides rich in Zinc has increased its content in soils with agricultural use (Singh et al., 2017). Agrochemicals such as mancozeb, propineb, zinc thiazole, zinc sulfates, zinc chelates, zinc oxides and nitrates are used in agriculture in Colombia as fertilizers, defoliants, fungicides and bactericides (Benitez et al., 2009). Terán-Chaves et al. (2023) mention that bulb onion production in Colombia is characterized by high production costs resulting from the excessive use of agrochemicals and the low implementation of good agricultural practices. Alengebawy et al. (2021) also comment that heavy metals and pesticides head the list of toxic compounds present in the environmental supply that endanger ecosystems. This uncontrolled use of pesticides in agriculture causes their bioaccumulation in the links of the food chains and in addition, their residues remain in plant organs, soil, air, and water sources.
In Colombia, short-day bulb onion varieties are grown whose genetic materials develop well in moderately cold climates, at an elevation of 800 to 2,500 m, a temperature of 16 to 27ºC, with high luminosity, wide difference in day and night temperature, and relatively low humidity during the day and night (Terán-Chaves et al., 2023). One of the most commonly planted hybrids is Yellow granex, which, throughout the crop cycle, presents variable zinc content in the tissues, with a maximum value in leaves and bulbs of 393.6 and 415.2 mg kg-1, respectively, at 95 days after sowing (DAS). Additionally, in roots, the Zn content showed an upward trend throughout the crop cycle, reaching its maximum value (343.3 mg kg-1) at the time of harvest (170 DAS) (Casierra-Posada and Vargas, 2015).
In sweet basil plants (Ocimum basilicum L.), Mahmoudi et al. (2021) found that exposure of plants to toxic levels of Zn induced a reduction in dry matter production, root length, and leaf number. Also, in these plants, excess Zn reduces plant growth, the content of photosynthetic pigments, phenols, and flavonoids. Additionally, plant growth was negatively affected and the appearance of brown roots with abnormal appearance was induced in white mustard (Sinapis alba L.) plants grown under conditions of excess Zn. In this case, growth, dry weight, photosynthetic activity and pigment content are reduced. However, there is also a slight increase in malondialdehyde content, with no appreciable changes in membrane permeability, perhaps due to an increase in proline content (Repkina et al., 2023).
Oprea et al. (2022) estimated the content of four different heavy metals in Allium cepa relative to the total metals contained in the soil. They found the highest correlation coefficients for Cd, Zn, Cu and Pb in onion bulbs. In addition, when Allium cepa plants are exposed to high Zn contents in the substrate, concentrations of Fe, Cu, Mn, Ca and Mg in the leaves are reduced (Ajakaiye and Greig, 1976). Sun et al. (2019) reported that treatment of Allium cepa plants with ZnO nanoparticles appreciably affects cell membrane integrity, metabolic activity, production and accumulation of reactive oxygen species, and leads to DNA alterations, chromosomal aberrations and the cell cycle affectation. These authors also found high Zn contents in the cytoplasm and nucleus, with a higher zinc content in the nucleus than in the cytoplasm. Based on these arguments, the objective of the present study was the evaluation of growth in bulb onion plants exposed to high Zn contents in nutrient solution under greenhouse conditions.
MATERIALS AND METHODS
The study took place in greenhouse conditions at the School of Agricultural Sciences of the Universidad Pedagógica y Tecnológica de Colombia in Tunja, Colombia, located at 2,690 m a.s.l. The environmental conditions during the experiment were: average temperature 17.1±8.3ºC, relative humidity 70.8±8.9% and average photosynthesis photon flux density (PPFD) of 1,871.4 μmol m-2 s-1±507.5, from natural light through glass sheets. Seedlings of bulb onion (Allium cepa L.) hybrid Yellow granex F1 were used. The seeds germinated in peat (TS1 876 Klasmann, Agriandes-Daymsa, Bogotá, Colombia) 10 days after sowing and were transplanted 56 days after germination to 500 mL glass containers, one plant per container.
Methods and evaluated variables
As a substrate for plant growth, a complete nutrient solution was prepared from simple sources and 450 mL of that solution were placed in each container, with the following composition (in g L-1): nitrogen (ureic) 0.4; phosphorous: 0.03; potassium: 0.05; calcium: 0.0005; magnesium: 0.0013; sulfur: 0.00137; boron: 0.0002; copper: 0.00014; iron: 0.00012; manganese: 0.0013; molybdenum: 0.00005 and zinc: 0.0002. According to the amount of zinc present in the nutrient solution, an amount was added to complete the value of the metal with which the plants would be treated, whose doses were: 0 (Control), 20, 40, and 80 mg L-1 of Zn. ZnSO4·7H2O (Merck® KGaA, Darmstadt, Germany) was used as a Zn source.
In the treatments, including the control, the pH of the solution was adjusted to 6.0 by adding NaOH or HCl 0.1 N. The glass containers were covered with aluminum foil to block light to the root zone and thus limit the growth of algae in the solution. To avoid hypoxia conditions, the solution was aerated throughout the test time with air pumps.
The plants of this bulb onion hybrid mature between 100-110 days after transplantation (DAT) and are harvested between 140-180 DAT; however, in the present study, the plants were harvested 54 DAT, since, at that time, the plants treated with the highest dose of Zn showed severe damage that endangered their survival. When removing the plants from the containers, measurements representing the dependent variables were recorded. The total length of the roots was measured by sectioning each of the roots of the plant and placing these in a line. The dry weight of the plant tissues was determined by drying in an oven at 70°C to constant weight. The leaf area was measured using a LI-COR® 3000A leaf area meter (LI-COR, Lincoln, NE). To record dry matter partitioning in each of its organs, each plant was sectioned into roots, bulb, pseudostem and leaves, and dried independently at 70ºC to constant weight, and then the percentage weight ratio of each organ was calculated based on the total dry matter of the plant. The content of total soluble solids in the bulb juice was determined using a PCE-DRC 2 refractometer (PCE GmbH, Meschede, Germany). Twice a week the decrease in the nutrient solution was recorded, new solution was then added until the original volume (450 mL) was completed; this added volume was recorded as the water uptake. Based on the water uptake and the dry weight per plant, the water use efficiency (WUE) was calculated. The values of absolute growth rate (AGR) and specific leaf area (SLA) were calculated with the methodology proposed by Hunt (1990).
Experimental design
A completely randomized statistical design was used, with 20 replicates per treatment and one seedling as the sampling unit. The results obtained were subjected to a classical analysis of variance, using an ANOVA table. The difference between means was calculated with Tukey's Honestly-significant-difference (HSD) test (P<0.05). Statistical analyses were performed with version 26 of IBM® SPSS® Statistics (Statistical Product and Service Solutions, Chicago, IL). The figures are presented in bar format with their respective standard deviation and the result of the Tukey test.
RESULTS AND DISCUSSION
The fresh weight of the bulb 54 DAT decreased significantly with the applications of Zn to the nutrient solution. The decrease compared to control plants was 87.6, 92.9 and 95.6% when plants were exposed to 20, 40 and 80 mg L-1 Zn, respectively (Tab. 1). Additionally, the diameter of the bulb at the end of the trial (54 DAT) was significantly reduced as a result of exposure to Zn. Plants exposed to 20, 40 and 80 mg L-1 of Zn showed a decrease in this variable of 67.3, 75.1 and 82.4%, respectively, compared to control plants (Tab. 1).
Table 1. Variables related to growth in plants of bulb onion (Allium cepa L.) hybrid Yellow granex F1 exposed to excess Zn in the nutrient solution (dose in mg L -1 ).
| Variable | 0 | 20 | 40 | 80 |
|---|---|---|---|---|
| Bulb fresh weight (g) | 40.58±23.21 a | 5.03±1.21 b | 2.87±2.16 b | 1.76±2.07 b |
| Leaf area (mm2) | 2,008.41±372.77 a | 191.17±83.61 b | 28.89±12.95 b | 31.95±9.33 b |
| Bulb diameter (cm) | 4.42±0.69 a | 1.44±0.23 b | 1.10±0.43 b | 0.77±0.49 b |
| Total root length (cm) | 10,109.75±3582.46 a | 813.50±370.21 b | 247.00±133.53 b | 301.66±121.80 b |
| Specific leaf area (mm2 mg-1) | 3.15±0.36 a | 2.42±0.55 a | 1.15±0.44 b | 1.25±0.14 b |
Means with different letters in the row indicate significant differences according to Tukey's HSD test (P<0.05) (n=20).
Oprea et al. (2022) found that in Allium cepa plants treated with different heavy metals, Zn was the metal registered in greater quantity in the bulbs, with a range of 1.0 to 11.7 mg kg-1, followed by Cu, Pb and Cd, indicating that the reduction in growth is caused by the excess of Zn accumulated in the cataphylls of the bulb. It has also been shown that plants exposed to high Zn content in the substrate show significant callose deposit in the apical meristem of the root, which can contribute to the inhibition of growth of plant organs, since cell wall expansion is decreased and symplastic transport is reduced (Feigl et al., 2015). In addition, high concentrations of pectin have also been recorded when plants are subjected to high Zn contents in the substrate. In these cases, callose and pectin appear to act in a complementary manner, so the increase in pectin binds to the high levels of Zn in the cell wall, with the callose deposit immobilizing it, so that Zn cannot enter the cytoplasm, thus inhibiting growth (Feigl et al., 2019; Kaur and Garg, 2021).
Kaur and Garg (2021) mention that excess Zn can increase the permeability of the membranes. The swelling of membranes in the thylakoids separates the sites where the complexes are found, thus decreasing the efficiency in the energy transfer and leading to nutritional imbalances in the tissues. Lipid peroxidation, membrane damage, alteration of proteins and genetic material in plants are other significant consequences caused by excess Zn, leading to irreparable metabolic dysfunction and severely affected cell viability. In the present study, metabolic alterations occurred in the plants that affected growth of the bulb represented by its fresh weight and diameter, given that the main sinks in bulb onion plants are the leaf blades, made up of cataphylls, which form the bulb (Casierra-Posada and Vargas, 2015).
No statistically significant difference was found in the measurements of dry matter partitioning; however, the excess of Zn in the nutrient solution had a statistically significant effect on the accumulation of total dry weight per plant; doses of 20, 40 and 80 mg L-1 of Zn induced a reduction of 87.3, 94.4 and 95.6 %, respectively, of this variable in relation to the control plants (Fig. 1). This shows the low tolerance of bulb onion plants to excess Zn in the substrate; the reduction in the production of dry biomass was so significant that the survival of the plants until the end of their growth cycle was endangered and an acceptable yield was not realized. This agrees with Ajakaiye and Greig (1976), who state that Allium cepa is a species very sensitive to excess Zn.
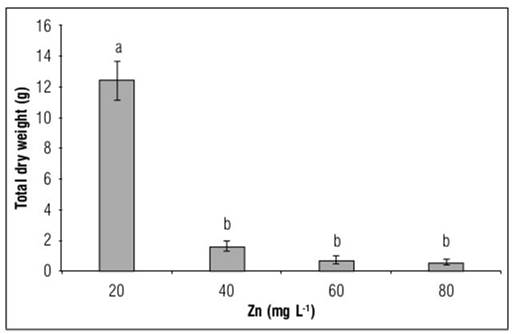
Figure 1. Total dry weight in Yellow granex F1 hybrid bulb onion plants (Allium cepa L.) exposed to excess Zn in the nutrient solution. Means with different letters indicate significant differences according to Tukey's HSD test (P<0.05) (n=20±SE).
As in the present study, the addition of 40 and 80 mg L-1 of Zn to a nutrient solution in which spinach plants (Spinacia oleracea L.)plants grew, induced a reduction in dry weight in plants of 75.6 and 91.2%, respectively, in relation to the control plants. Casierra-Posada et al. (2010) found that in broccoli (Brassica oleracea var. Italica Plenck) plants, zinc added to the soil in concentrations of 50, 100 and 200 mg kg-1 reduced the total dry weight of the treated plants by 25.9, 42.8 and 47.8%, respectively in relation to the control plants. Likewise, in strawberry plants (Fragaria x ananassa (Weston) Duchesne ex Rozier), Casierra-Posada and Poveda (2005) found that the addition of 350 mg kg-1 of Zn to the soil reduced the dry matter content to close to half that of the control plants.
The effects of Zn toxicity are more drastic when the metal is added to a nutrient solution than when applied to soil. This happens because the nutrient solution lacks organic matter. Hassan et al. (2022) mention that the availability of Zn in the soil solution is significantly determined by factors such as soil pH, organic matter content, moisture, type and composition of clays, and the content of carbonates. Hussain et al. (2022) add that the possibility of exchange and bioavailability of Zn depend largely on the pH value in the soil, since this is where adsorption and desorption of organic matter take place. Additionally, the development of poorly soluble Zn compounds, such as sulfides, is favored by the oxidation of organic matter in the case of zinc sedimentation, which also decreases the availability of zinc to plants.
This present study showed that exposure to high doses of Zn reduced the plant’s Absolute Growth Rate in relation to the control plants by 89.5, 96.8 and 97.4%, with exposure to doses of 20, 40 and 80 mg L-1 of Zn, respectively (Fig. 2).
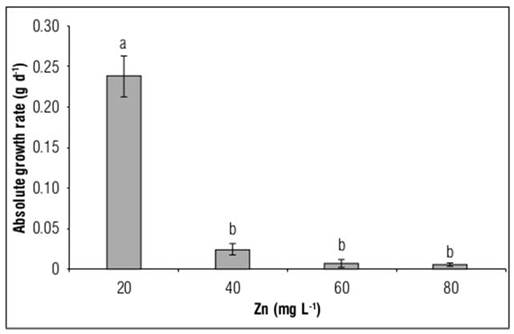
Figure 2. Absolute growth rate (AGR) in Allium cepa hybrid Yellow granex F1 exposed to excess Zn in the nutrient solution. Means with different letters indicate significant differences according to Tukey's HSD test (P<0.05)(n = 20±SE).
Mahmoudi et al. (2021) exposed sweet basil (Ocimum basilicum L.) seedlings to concentrations between 1 and 5 mM ZnSO4 in a nutrient solution. The seedlings treated with Zn experienced oxidative stress and prolonged lipid peroxidation which led to oxidative damage to the membranes, causing a significant reduction in seedling growth. Additionally, Kaur and Garg (2021) mention that the reduction of growth in plants exposed to relatively high Zn contents is linked to a significant decrease in dry weight, growth of aerial and root parts, number of leaves, water content in tissues and chlorophyll, to unbalanced mineral nutrition, and to decreased photosynthetic and respiratory rates.
Our study showed that increasing doses of Zn in the nutrient solution induced an increase in the content of soluble solids in the juice of the bulb, with statistical differences between treatments. Thus, the addition of 20, 40 and 80 mg L-1 of Zn, increased the value of this variable by 17.8, 34.3 and 50%, respectively, with respect to the average values recorded in the control plants (Fig. 3).
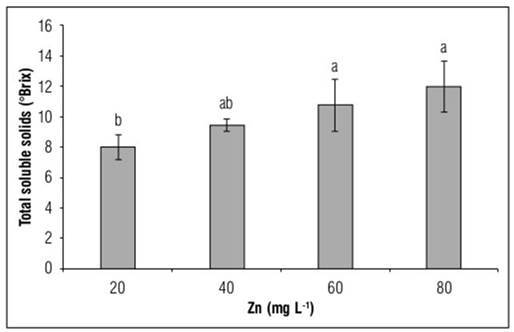
Figure 3. Total soluble solids in Yellow granex F1 hybrid bulb onion plants (Allium cepa L.) exposed to excess Zn in the nutrient solution. Means with different letters indicate significant differences according to Tukey's HSD test (P<0.05) (n = 20±SE).
Abou El-Nasr et al. (2021) observed that the foliar spraying of common grape vine (Vitis vinifera L.) plants with 25 mg L-1 of ZnO in two consecutive years increased the values of total soluble solids (TSS) by 18.1 and 19.5ºBrix, respectively. On the other hand, the minimum values of TSS, 15 and 15.6ºBrix, were recorded in berries obtained from the control treatment during the first and second season, respectively. This evidences the role of Zn in the transfer and synthesis of proteins and carbohydrates and in maintaining the structural stability of cell membranes, in addition to its role in many biochemical pathways. These authors mention that their results coincide with those obtained by other authors, who used similar methodologies for the application of Zn in grape and pomegranate plants. Rafie et al. (2017) foliar sprayed Allium cepa plants, using different sources of Zn. They found that all sources of Zn significantly increased the concentration of this metal in the bulb. Additionally, in the Perimavera cultivar, all sources of Zn increased the concentration of pyruvic acid in the bulb, but in the Behbahan cultivar, only some Zn complexes were effective in increasing the concentration of pyruvic acid in the bulb.
On the contrary, the addition of 350 mg kg-1 of Zn to the soil in which strawberry plants (Fragaria x ananassa (Weston) Duchesne ex Rozier) grew reduced the TSS content in the fruits by 21.8% (Casierra-Posada and Poveda, 2005). These authors also mention that Zn toxicity negatively affects transport by the phloem, so that carbohydrates accumulate in the primary leaves and cannot be translocated to the organs of demand, causing a strong reduction in the basipetal transport of assimilates and the consequent accumulation of sucrose and starch in the leaves. In this sense, the increase in the content of TSS in the present study can be attributed to the morphology of the bulb onion plant, since the TSS measurements were made in the juice of the bulb that is made up of cataphylls, which constitute the base of the leaves and are the strongest sink of these plants. In addition, according to Ajakaiye and Greig (1976), the onion bulb is an important sink; they found more Zn in the bulbs than in the leaves under conditions of increasing doses of Zn applied to the soil from two different sources.
As a consequence of the addition of Zn to the nutrient solution in the present study, the water uptake by the plants was significantly reduced. Water uptake decreased 86.7, 87.8 and 88.7% compared to control plants, with exposure of plants to doses of 20, 40 and 80 mg L-1 Zn, respectively (Fig. 4).
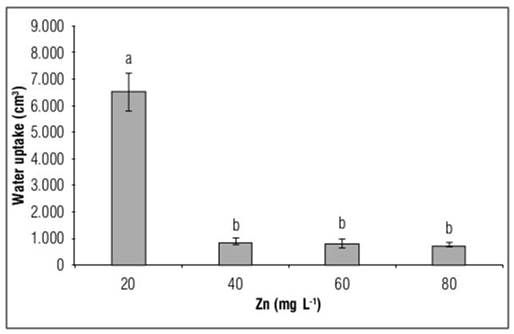
Figure 4. Water uptake in Yellow granex F1 hybrid bulb onion (Allium cepa L.) plants exposed to excess Zn in the nutrient solution. Means with different letters indicate significant differences according to Tukey's HSD test (P<0.05) (n=20±SE).
Casierra-Posada et al. (2012) found that the application of 40 and 80 mg kg-1 of Zn to the soil decreases the water uptake in spinach plants (Spinacia oleracea L.) by 61.8 and 66.5%, respectively with respect to control plants. The excess of Zn has a rather severe effect on the roots, with rotting, decreased elongation, and reduction in the number of roots, absorption area and hydraulic conductance, according to Kaur and Garg (2021). Likewise, the photosynthetic activity of plants exposed to high Zn contents was reduced as a consequence of the effect of excess Zn on the hydraulic conductivity of the roots. This effect is possibly due to structural changes in the xylem, which negatively affect photosynthetic activity by inducing a lack of water coupled with a reduction in stomatal conductance (Cakmak et al. 2023). Additionally, Mahmoudi et al. (2021), in sweet basil (Ocimum basilicum L.) plants, found that the elongation that takes place in the radicle is more sensitive to excess Zn than the germination of the seeds themselves. In this sense, the inhibition of the growth of the root zone as a consequence of the excess of Zn can be the result of the inhibition of mitosis in the tips of the roots, of the reduction of the elongation and also of a decrease in the cell viability in the zone of elongation in the roots. Additionally, these effects could be accentuated due to root hardening from the excess Zn, which as a result of reduced root growth, would consequently induce a reduction in water uptake.
WUE in the present study was reduced in all treatments with exposure to high doses of Zn, with statistically significant differences. However, this reduction was more significant at 40 mg L-1 Zn. Thus, exposure to this metal reduced WUE by 54.6 and 60.5% compared to control plants, when plants were exposed to 40 and 80 mg L-1 Zn, respectively (Fig. 5).
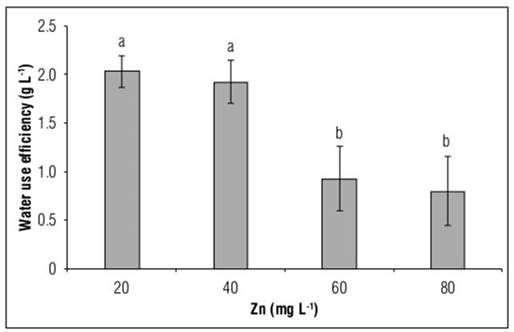
Figure 5. Water use efficiency (WUE) in Yellow granex F1 hybrid bulb onion (Allium cepa L.) plants exposed to excess Zn in the nutrient solution. Means with different letters indicate significant differences according to Tukey's HSD test (P<0.05) (n=20±SE).
Zaheer et al. (2020) observed that the application of Zn (10 mg L-1) improved WUE by 126% in rapeseed plants (Brassica napus L.)grown under stress due to excess Cr. An exogenous supply of Zn has been shown to improve WUE and nutrient uptake, enhancing plant growth by controlling the process of photosynthesis and transpiration (Hussain et al., 2018). On the other hand, Repkina et al. (2023) observed a rather drastic decrease in WUE in white mustard plants (Sinapis alba cv. Belgia) exposed to 150 mg kg-1 Zn in relation to control plants, while in plants that grew exposed to Zn concentrations below that value, no significant differences were induced. Additionally, these authors observed that the relative water content (RWC) was reduced when plants grew exposed to 100-150 mg kg-1 Zn.
Subba et al. (2014) mention that when plants are exposed to high Zn contents, there is a reduction in stomatal conductance. Metals usually induce closure of stomata, changes in membrane permeability, destruction of guard cells and a very high increase in abscisic acid content, which results in partial or total closure of stomata. However, this reaction in the plant may be an adaptive mechanism to maintain the relative water content in the tissues (Repkina et al. 2023). Our study observed the drastic impact of excess Zn in the substrate, which decreased water uptake and WUE, and consequently, significantly reducing the production of dry matter, thus limiting not only the survival of plants but also the size of the bulbs, which do not reach the quality for marketing, as observed in table 1.
In our study, the roots of plants exposed to excess Zn grew less than in control plants, with statistical differences. Thus, doses of 20, 40 and 80 mg L-1 of Zn in the nutrient solution induced a reduction of 91.9, 97.5 and 97.0%, respectively, in this variable (Tab. 1). In agreement with the findings of our study, Palacio et al. (2005) found that in Allium cepa plants, zinc behaved as a nutrient when plants were exposed to a concentration of up to 1.0 mg L-1, but concentrations above this produced a toxic effect and induced a 50% reduction in the average length of the roots. Sun et al. (2019) exposed Allium cepaplants to ZnO nanoparticles added in solution (Zn2+ released from 5 to 50 μg mL-1 of ZnO) for 36 h and found reduced root growth by 24.2 to 36.1%, with the tips of the roots swollen, soft and friable. In addition, the appearance of a transparent zone and breaks in the elongation zone were observed when the plants were exposed to 50 μg mL-1 of ZnO. Additionally, according to Cakmak et al. (2023), when plants are exposed to high contents of Zn, the toxic effect caused by this element can be induced and is easily evident in non-tolerant plants, in which the inhibition of root elongation is a quite sensitive indicator. This corroborates the findings of Ajakaiye and Greig (1976), who mention that Allium cepa is a plant very sensitive to excess Zn.
The exposure of Allium cepa plants to toxic Zn contents in the solution, taking ZnO as the source of the metal, negatively altered the integrity of cell membranes, allowing ZnO nanoparticles to easily enter the cells and thus reducing the growth of bulb onion plants (Sun et al., 2019), which is in agreement with the findings of Ghosh et al. (2015). Thus, despite the fact that zinc is a fundamental element for plants, for its role in cell division, expansion and accumulation of dry matter of leaves and mitosis in the apices of the roots, the excess of this metal in the substrate severely affects growth. Bulb onion plants are very sensitive to excess Zn, more so than potato plants(Solanum tuberosum L.)(Ajakaiye and Greig, 1976).
In the present study, plants exposed to high doses of Zn in the nutrient solution showed a significant reduction in leaf area compared to control plants, with statistically significant differences. Plants exposed to 20, 40 and 80 mg L-1 Zn showed a reduction in leaf area of 90.4%, 98.5 and 98.4%, respectively (Tab. 1). In this regard, Casierra-Posada et al. (2010) found that plants ofbroccoli(Brassica oleracea var. Italica Plenck) subjected to 50, 100 and 200 mg kg-1 of zinc showed a reduction of 19.6, 34.1 and 39.8% in the leaf area, respectively, compared to the control plants, without significant difference among all treatments containing Zn, but with significant differences between these treatments and the control plants. Similarly, Casierra-Posada et al. (2012) observed that Spinacia oleracea plantssubjected to 40 and 80 mg L-1 Zn showed reduced leaf area by 78.8% and 94.5%, respectively, compared to the control plants. Kaur and Garg (2021) in their review, mention that excess Zn results in decreased leaf area. Additionally, stomatal limitations may be presented, further accentuating the flow of CO2 in the mesophyll, which hinders the entry of CO2 into the chloroplasts, consequently causing a negative effect on carbon fixation during photosynthesis. This was observed in the present study, with excess Zn inducing a fairly important reduction in the production of dry matter per plant (Fig. 1) and a reduction in the leaf area (Tab. 1).
In the present study, the specific leaf area (SLA), expressed in mm2 mg-1, of plants that grew exposed to excess Zn in the nutrient solution, showed a reduction of 23.1, 63.3 and 60.1%, when 20, 40 and 80 mg L-1 of Zn were added, respectively. These values were recorded in comparison with the control plants, with statistically significant differences (Tab. 1). In agreement, Casierra-Posada et al. (2012), in spinach plants (Spinacia oleracea L.), found an increase in specific leaf weight of 60.3 and 82.7% when the plants were exposed to 40 and 80 mg L-1 of Zn, respectively with respect to control plants. This evidences that as the concentration of Zn in the solution in which the plants grew increased, the weight of the leaf per unit area increased. This is in line with our findings.
Liu et al. (2009) mention that the specific leaf area is not only a measure to evaluate leaf function and resource acquisition and use but is also a highly relevant record in the simulation of morphogenesis, ecological adaptation, and nutrient uptake in plant populations. In addition, it is a transcendental physiological index to evaluate the growth of plants. These authors also mention that, under normal conditions, without a demanding environmental offer, species that register low values in the SLA generally have longer-lived leaves which contain higher amounts of ribulose bisphosphate carboxylase/oxygenase (Rubisco) compared to species that present high values of SLA, thus achieving greater photosynthetic capacity, biomass production and therefore higher yield.
Plants with higher specific rhizome length and SLA values exhibit rapid growth and generally grow in resource-rich sites (Shovon et al., 2020). On the other hand, morphological and physiological adaptations in the leaf blades are decisive for plants to have the ability to use the limited resources available in a demanding environmental offer marked by some type of abiotic stress (Zhou et al., 2020). Thus, plants exposed to stress conditions tend to reduce SLA values, as reported by Hamal and Chettri (2022) who, in various plant species exposed to conditions of toxicity by Cu, Pb or Zn, found that in all species the SLA value decreased in contaminated sites compared to control plants. In addition, these authors mention that the reduction in SLA values leads to a reduction in water loss and transpiration, which is considered an adaptive strategy of plants to increase their tolerance to stress. In this sense, our study evidenced that the reduction in the values of the SLA in an inversely proportional manner with the increase in the content of Zn in the solution was an adaptation mechanism to withstand as far as possible the stress due to excess Zn. However, this adaptive strategy was not enough to ensure that the plants completed their life cycle, since the effect on other variables involved in growth such as leaf area, total dry weight, water uptake and WUE was too drastic. Allium cepa plants are quite sensitive to stress due to high Zn contents, as mentioned by Ajakaiye and Greig (1976).
CONCLUSION
The high sensitivity of bulb onion plants to excess Zn in the substrate was observed. The effect of excess Zn is more drastic when plants are grown in nutrient solution than when grown in soil, since, in the soil, organic matter partially buffers the damaging effect of Zn, especially in the roots. Therefore, in soils poor in organic matter, growers must be careful about applying pesticides and fertilizers rich in zinc, since excess Zn can induce deficiencies of other elements such as phosphorus, magnesium and manganese, since Zn competes and interferes with their absorption by the roots. All variables involved in plant growth were severely affected by excess Zn in the solution. In this way, the production of dry mass is reduced as a result of the decrease in water uptake, in the WUE and in the leaf area. Additionally, the average values of these variables are strongly reduced by the decrease in root growth.















