INTRODUCTION
Lima bean (Phaseolus lunatus L.) is the second most important species of the genus Phaseolus, after the common bean (P. vulgaris). This crop is important in Brazil due to its protein content, serving as a food source for the Brazilian population (Santos et al., 2009; Araujo et al., 2015). In addition, this crop is a source of income for small producers in the northeastern region of the country and the state of Rio Grande do Sul (Franco et al., 2002). Additionally, although there is disagreement about its use in agriculture (Costa and Durigan, 2010), Leucaena leucocephala (Lam.) de Wit (leucaena) is a protein-rich legume used for animal production, for the development of silvopastoral and agroforestry systems in several Latin American countries such as Colombia, Panama, Costa Rica, Mexico, among others (Murgueitio et al., 2016), as well as for the recovery of degraded areas and reforestation in tropical soils (Barreto et al., 2010; Bueno and Camargo, 2015; Nicodemo et al., 2018; Kant et al., 2019).
In recent years, there has been growing interest in the production of food for human and animal food in a sustainable manner, with lower environmental impact and greater food security, especially with regard to the development of ecological intensification strategies, such as the efficient use of nutrients, biostimulant products for plant growth, the reduction of the need for disease and pest control, among others (Tittonell, 2014; Canellas et al., 2015). Plant growth biostimulants are substances or microorganisms that, when applied or inoculated in the rhizosphere or in plants, improve nutrient absorption, nutritional efficiency, tolerance to abiotic stress and crop quality. Examples include humic substances (HS) and plant growth-promoting rhizobacteria (PGPR) (Yakhin et al., 2017).
HS are the colloidal fraction of soil organic matter, being classified as humic acids (HA), fulvic acids (FA) and humines based on their solubility in acid or alkaline pH. Sources of HS include vermicompounds, sewage, peat, lignocellulose residues from refineries for ethanol production and coal production residues such as lignites and leonardite (Silva et al., 2000; Canellas et al., 2000; Canellas and Façanha 2004; Aguirre et al., 2009; Giannouli et al., 2009; Canellas and Olivares, 2014; Cubillos-Hinojosa et al., 2015; Spaccini et al., 2019). These latter sources are considered rich in HA and fulvic acids FA, obtained by the classical method of extraction with alkaline solutions (Senesi et al., 2007; Chassapis and Roulia, 2008; Giannouli et al., 2009), with leonardite the most used source for HS extraction in the HS-based product manufacturers industry.
The bioactivity of HS in the promotion of plant growth has been widely reported in several studies, which show that HS stimulates plant growth and development, induction of root proliferation by modifying root system architecture, leaf development, increased absorption of nutrients and regulation of enzymes important for plant metabolism, such as H+-ATPase, VATP-ase, nitrate reductase, and auxinic (Chen and Avaid, 1990; Pinton et al., 1992; Façanha et al., 2002; Nardi et al., 2005; Chen et al., 2004; Zandonadi et al., 2007; Dobbss et al., 2010; Trevisan et al., 2010; Zandonadi and Busato, 2012; Zandonadi et al., 2013, Zandonati et al., 2014; Canellas and Olivares, 2014; Canellas et al., 2015; Shah et al., 2018).
These effects of HS on plant development depend on source, dose and genotype of plant (Vaughan and Malcolm, 1985; Rodda et al., 2006; Zandonadi et al., 2014). In lima bean plants, there are only reports of the effects of HS on the modification of potassium absorption kinetics, assimilation of mineral elements such as phosphorous and nitrogen, plant growth and nutrient concentration in common beans (P. vulgaris) (Rosa et al., 2009; Aydin et al., 2012). However, there were no reports on these effects in P. lunatus and L. leucocephala.
Plant growth-promoting rhizobacteria (PGPR) are a very diverse group of bacteria that have the capacity to promote plant growth (Kloepper and Schroth, 1978) by means of several mechanisms such as: biological nitrogen fixation (BNF) (Iniguez et al., 2004; Montañez et al., 2009), the production of auxins, cytokinins, gibereline strain and ethylene inhibition (Arshad and Frankenberger, 1992), antagonism against phytopathogens by the production of siderophores (Scher and Baker, 1982), the induction of systemic acquired resistance (Pieterse et al., 2014), or increased availability of nutrients such as phosphorus (Sturz et al., 2000; Sessitsch et al., 2002). These effects of PGPR on the growth of common bean, lima bean and leucaena plants have been documented in several studies, with some strains of PGPR used in the formulation of commercial inoculants (Hungria et al., 2000; Hungria et al., 2003; Yadegari et al., 2008; Bueno and Camargo 2015; Aguirre-Medina et al., 2015; Cubillos-Hinojosa et al., 2019; Cubillos-Hinojosa et al., 2020; Cubillos-Hinojosa et al., 2021). In common bean, the co-inoculation of rhizobia and A. brasilense is recommended, generating an increase in nodulation and crop yield (Hungria et al., 2013). However, despite some studies showing that several cultivars of lima bean can establish symbiotic association with rhizobia efficient in the biological nitrogen fixation (Ormeño et al., 2007; Santos et al., 2009; Antunes et al., 2011; Santos et al., 2011; Servín-Garcidueñas et al., 2014; Duran et al., 2014; Ormeño-Orrillo et al., 2017; Cubillos-Hinojosa et al., 2021; Cubillos-Hinojosa et al., 2023), there are no reports of the use of co-inoculation rhizobia and A. brasilense in either lima bean and the same in leucaena plants.
The combined use of PGPR and humic substances (HS) is reported in some studies on corn, tomato, pineapple, and potato crops, showing increases in crop yield and stress mitigation in plants when compared with the isolated application (Melo et al., 2017; Ekin, 2019). However, there are no reports yet for fava beans and leucaena. In common bean plants (Phaseolus vulgaris L.), a reduction in water stress was observed with the combined application of HS extracted from vermicompost and co-inoculation of Rhizobium tropici strains BR322, BR520 and BR534 and Herbaspirillum seropedicae (Melo et al., 2017). Despite these findings, there is still a lack of studies on the effects of the combined application of HS and efficient rhizobia in the biological fixation of nitrogen co-inoculated with A. brasilense in lima bean and leucaena plants.
The objective of this research was to evaluate the effect of inoculation or co-inoculation of rhizobia and A. brasilense in combined application with humic substances (humic and fulvic acids, extracted from leonardite) on the growth promotion of lima bean and leucaena plants.
MATERIALS AND METHODS
During the summer in January and February (2019), two experiments were carried out with plants of P. lunatus and L. leucocephala in the greenhouse of the Department of Soils of the Federal University of Rio Grande do Sul (UFRGS), Brazil. Seeds and bacterial crops were processed at the Soil Microbiology Laboratory of the Faculty of Agronomy of UFRGS.
Seed disinfection
Seeds of “olho de cabrapreto” variety of lima bean (P. lunatus) plants were used, supplied by producers from northeastern Brazil. These seeds were disinfected by successive immersion in alcohol (70%) for 30 s, sodium hypochlorite (2.5%) for 30 s, followed by six consecutive washes with sterile distilled water (Vincent, 1970). Then, the seeds were sowed in plastic pots with a capacity of 1.5 L that had been washed and flamed with 99% ethyl alcohol previously. The pots were filled with a sterile mixture, vermiculite and sand in proportion (2:1) in autoclave.
For the experiment with leucaena plants (L. leucocephala), seeds from the same plant at the Agronomic Experimental Station (EEA) of the Federal University of Rio Grande do Sul (UFRGS) were collected. These seeds were scarified with sandpaper Nº 100 for 1 min, then disinfected by the Vincent (1970) method as previously described for lima bean. In this experiment, plastic pots with a capacity of 700 mL that had been previously disinfected with 99% ethyl alcohol were used, rinsed successively with sterile distilled water and filled with a mixture of vermiculite and sand in proportion (2:1) sterilized in autoclave. Three seeds were sown in each pot.
Production of the inoculums of the isolates and strains of growth-promoting rhizobacteria
For the experiment with lima bean plants, the rhizobia Plu03 and Plu14 isolates and the SEMIA 4077 strain (Rhizobium tropici CIAT 899) were evaluated. This strain, released by the “Ministério da Agricultura, Pecuária e Abastecimento - MAPA” of Brazil to produce commercial inoculants for common bean (Phaseolus vulgaris L.), was obtained from the “SEMIA” collection of rhizobia of the Departamento de Diagnóstico e Pesquisa Agropecuária (DDPA), Rio Grande do Sul State, Brazil. These isolates and strains were selected for their ability to establish symbiosis and fix nitrogen in a previous experiment in lima bean plants of the “olho de cabrapreto” variety (Cubillos-Hinojosa et al., 2021).
In the experiment with leucaena plants, the rhizobia studied were the isolate leu01 and strains SEMIA 4081 and SEMIA 6361, obtained from leucaena plants from the rhizobia collection of the DDPA. Both the isolate Leu01 and strains SEMIA 4081 and SEMIA 6361 were selected for their efficiency in BNF in a previous experiment (Cubillos-Hinojosa et al., 2020).
In the production of bacterial inoculums, the bacteria were inoculated separately in falcon tubes with 30 mL of liquid mannitol yeast culture medium (Vincent, 1970) and kept in an orbital incubator at 28°C±2 with agitation of 120 rpm until reaching a concentration of 108 cells/mL. In the case of Azospirillum brasilense, a commercial product containing the AbV5 and AbV6 strains of A. brasilense with concentration of 108 cells/mL was used.
Humic substances
Two commercial biostimulators rich in humic substances (HS) extracted from leonardite coal were used: one of the products was based on fulvic acids (FA) at a concentration of 1.35%, referred to as FA. The other product was based on humic acids (HA) at a concentration of 1.44%, referred to as HA. The HS were applied at the dose recommended by the manufacturer of 4 L ha-1, and 10 mL of a solution was added (7.5 mL L-1).
Design of the lima bean experiment in a greenhouse
A randomized complete block design with 23 treatments and five replications was established, with two isolates (Plu03 and Plu14) and the strain (SEMIA 4077) inoculated or co-inoculated with A. brasilense, an addition of mineral N (control) and the combined application of humic acids (HA) and fulvic acids (FA), as presented in table 1.
One of the non-inoculated control treatments received nitrogen (N) in the form of NH4NO3 at a dose equivalent to the addition of 100 kg ha-1 (control+N) and two treatments included the addition of N in conjunction with the HS (control+N+HA and control+N+FA) (Tab. 1). Nitrogen was added fractionally in 5 applications, with a solution (0.015 g L-1) of NH4NO3 added weekly in 10 mL pots.
The pots of the treatments inoculated with the rhizobia received 2 mL of bacterial broth at sowing. The treatments co-inoculated with rhizobia and A. brasilense and those inoculated with only A. brasilense received 1 mL of the commercial product containing the strains AbV5 and AbV6 of A. brasilense in the sowing.
Table 1. Identification and description of experiment treatments in lima bean plants.
| Treatment | Description |
|---|---|
| Control-N | Not inoculated, no N |
| Control+N | Not inoculated, with N |
| N+HA | Not inoculated, with N + humic acids |
| N+FA | Not inoculated, 100% N + fulvic acids |
| Az | Inoculation A. brasilense |
| Plu03 | Inoculation of rhizobia isolate Plu03 |
| Plu03+Az | Co- inoculation rhizobia isolate Plu03 and A. brasilense |
| Plu03+FA | Inoculation rhizobia isolate Plu03 + fulvic acids |
| Plu03+HA | Inoculation rhizobia isolate Plu03 + humic acids |
| Plu03+Az+FA | Co- inoculation rhizobia isolate Plu03 + A. brasilense + fulvic acids |
| Plu03+Az+HA | Co- inoculation rhizobia isolate Plu03 + A. brasilense + humic acids |
| Plu14 | Inoculation of rhizobia isolate Plu14 |
| Plu14+Az | Co- inoculation rhizobia isolate Plu14 and A. brasilense |
| Plu14+FA | Inoculation rhizobia isolate Plu14 + fulvic acids |
| Plu14+HA | Inoculation rhizobia isolate Plu14 + humic acids |
| Plu14+Az+FA | Co- inoculation rhizobia isolate Plu14 + A. brasilense + fulvic acids |
| Plu14+Az+HA | Co- inoculation rhizobia isolate Plu14 + A. brasilense + humic acids |
| SEMIA 4077 | Inoculation rhizobia SEMIA 4077 |
| SEMIA 4077+Az | Co- inoculation rhizobia SEMIA 4077 and A. brasilense |
| SEMIA 4077+FA | Inoculation rhizobia SEMIA 4077 + fulvic acids |
| SEMIA 4077+HA | Inoculation rhizobia SEMIA 4077 + humic acids |
| SEMIA 4077+Az+FA | Co- inoculation rhizobia SEMIA 4077 + A.brasilense + fulvic acids |
| SEMIA 4077+Az+HA | Co- inoculation rhizobia SEMIA 4077 + A.brasilense + humic acids |
HA: humic acids; FA: fulvic acids; Az: Azospirillum brasilense; N: nitrogen; Plu03 and Plu14: rhizobia isolates; SEMIA 4077: rhizobia strain.
The humic substances (HA and FA) were added 1 d before seeding and nitrogen in the form of NH4NO3 was added 3 d after seeding. All plants were irrigated with nutrient solution of Hoagland and Arnon (1950), modified by Silveira et al. (1998). One week after seeding, thinning was performed, leaving one plant per pot.
Design of the leucaena experiment in a greenhouse
The experimental design in randomized blocks with five replications was conducted. Twenty-three treatments were established, with one isolate Leu01 and two strains SEMIA 4081 and SEMIA 6361, separately or co-inoculated, with addition of N (control) and combined application with humic acids (HA) and fulvic acids (FA) (Tab. 2).
Table 2. Identification and description of experiment treatments with leucaena plants.
| Treatment | Description |
|---|---|
| Control-N | Not inoculated, no N |
| Control+N | Not inoculated, with N |
| N+HA | Not inoculated, with N + humic acids |
| N+FA | Not inoculated, 100% N + fulvic acids |
| Az | Inoculation A. brasilense |
| Leu01 | Inoculation of rhizobia isolate Leu01 |
| Leu01+Az | Co- inoculation rhizobia isolate Leu01 and A. brasilense |
| Leu01+FA | Inoculation rhizobia isolate Leu01 + fulvic acids |
| Leu01+HA | Inoculation rhizobia isolate Leu01 + humic acids |
| Leu01+Az+FA | Co- inoculation rhizobia isolate Leu01 + A. brasilense + fulvic acids |
| Leu01+Az+HA | Co- inoculation rhizobia isolate Leu01 + A. brasilense + humic acids |
| SEMIA 4081 | Inoculation rhizobia SEMIA 4081 |
| SEMIA 4081+Az | Co- inoculation rhizobia SEMIA 4081 and A. brasilense |
| SEMIA 4081+FA | Inoculation rhizobia SEMIA 4081 + fulvic acids |
| SEMIA 4081+HA | Inoculation rhizobia SEMIA 4081 + humic acids |
| SEMIA 4081+Az+FA | Co- inoculation rhizobia SEMIA 4081 + A. brasilense + fulvic acids |
| SEMIA 4081+Az+HA | Co- inoculation rhizobia SEMIA 4081 + A. brasilense + humic acids |
| SEMIA 6361 | Inoculation rhizobia SEMIA 6361 |
| SEMIA 6361+Az | Co- inoculation rhizobia SEMIA 6361 andA. brasilense |
| SEMIA 6361+FA | Inoculation rhizobia SEMIA 6361 + fulvic acids |
| SEMIA 6361+HA | Inoculation rhizobia SEMIA 6361 + humic acids |
| SEMIA 6361+Az+FA | Co- inoculation rhizobia SEMIA 6361 + A.brasilense + fulvic acids |
| SEMIA 6361+Az+HA | Co- inoculation rhizobia SEMIA 6361 + A.brasilense + humic acids |
HA: humicacids; FA: fulvic acids; Az: Azospirillum brasilense; N: nitrogen; Leu01: rhizobia isolate; SEMIA 4081: rhizobia strain; SEMIA 6361: rhizobia strain.
One of the non-inoculated control treatments received N in the form of NH4NO3 at a dose equivalent to the addition of 100 kg ha-1 (control+N) and two treatments included the addition of N in conjunction with the HS (control+N+HA and control+N+FA) (Tab. 2). Nitrogen was added weekly in 6 applications, with 10 mL of a solution (1.43 g L-1) of NH4NO3 placed in the pots.
The treatments that were inoculated with rhizobia received 2 mL of bacterial broth in the seeds sowing. The treatments co-inoculated rhizobia and Azospirillum as well as the treatment inoculated only with A. brasilense received 1 mL of the commercial product containing the strains AbV5 and AbV6 of A. brasilense atsowing. The humic substances (HA and FA) were added 1 d before seeding and nitrogen in the form of NH4NO3 was added 3 d after seeding. All plants were irrigated with nutrient solution of Sarruge (1975). One week after, thinning was performed, leaving one plant per pot.
Measured variables
After 45 d of cultivation, the lima bean and leucaena plants were collected, and the aerial part of the root system was separated. Shoot dry mass (SDM), root dry mass (RDM), mass of dry nodules (MDN), and subsequently, total accumulated N (Nac) in shoots and relative efficiency index (REI) of biological nitrogen fixation of rhizobia were determined according to Brockwell et al. (1966). The aerial part was packed in paper bags and dried in the forced circulation oven at 65°C for 3 d and then ground for the quantification of N by the method described by Tedesco et al. (1995). All roots were washed to remove particles from adhered substrate and dried with an absorbent paper towel to remove excess water. Then, the nodules of the treatments inoculated with rhizobia in lima bean were removed from the roots and dried in paper bags under the same conditions and weighed to determine the MDN. In leucaena plants nodules of the treatments inoculated with rhizobia were removed and quantified.
The relative efficiency index (REI) in biological nitrogen fixation was calculated using the equation (1) (Brockwell et al., 1966)
where, NT was total nitrogen from the plant of the inoculated treatment, NT-N the total nitrogen non-inoculated control without nitrogen and, NT+N the total nitrogen of the non-inoculated control with nitrogen supplementation.
RESULTS AND DISCUSSION
Effect of the combined application of HS and rhizobia inoculated and co-inoculated with A. brasilense on lima bean plants
The results of the variables evaluated in the growth promotion of lima plants in response to the treatment with rhizobia inoculated and co-inoculated with A. brasilense in conjunction with HS are presented in table 3.
For the dry mass of the plants, the treatments Plu14+Az+FA and Plu14+Az+HA showed the highest increases (8.49 and 8.61 g, respectively), compared with the other treatments and the control treatment (control+N), which obtained 4.68 g (Tab. 3). The greatest increases were when plants were co-inoculated with rhizobia and A. brasilense in combined application with HS (treatments Plu03+Az+FA; Plu03+Az+HA; SEMIA 4077+Az+FA and SEMIA 4077+Az+HA), followed by plants inoculated with rhizobium with HS (Plu03+HA; Plu03+FA; Plu14+HA; Plu14+FA; SEMIA 4077+HA; SEMIA 4077+FA). There were no differences with plants co-inoculated with rhizobia and A. brasilense and plants treated with N with HS (N+HA and N+FA). All these treatments were superior to the control+N treatment and the isolated inoculation of rhizobia except for N+HA and N+FA treatments, which were similar to the treatment inoculated with rhizobia Plu03 but superior to the control+N treatment.
Table 3. Effect of inoculation and co-inoculation of rhizobia (Plu03, Plu14 and SEMIA 4077) and A. brasilense (Az) combined with humic acids (HA) and fulvic acids (FA), in the promotion of growth of lima bean plants.
| Treatments | SDM | RDM | MDN | Nac |
|---|---|---|---|---|
| (g) | (mg) | |||
| Control+N | 4.68 f | 1.20 f | 0 h | 174.30 h |
| Plu14 + Az+ HA | 8.61 a | 2.18 a | 1,298 a | 461.97 a |
| Plu14 + Az+ FA | 8.49 a | 2.06 a | 1,276 a | 429.92 b |
| SEMIA 4077 + Az+ HA | 7.90 b | 1.87 b | 984 c | 385.66 c |
| Plu03 + Az+ HA | 7.85 b | 2.07 a | 1,140 b | 422.66 b |
| SEMIA 4077 + Az+ FA | 7.85 b | 1.89 b | 980 c | 383.09 c |
| Plu03 + Az+ FA | 7.71 b | 2.06 a | 1,100 b | 383.43 c |
| Plu14 + HA | 7.22 c | 1.86 b | 1,106 b | 341.53 d |
| Plu14 + Az | 7.13 c | 1.69 c | 884 d | 339.19 d |
| Plu14 + FA | 7.04 c | 1.65 cd | 1,088 b | 330.14 d |
| Plu14 | 6.53 d | 1.41 e | 762 e | 292.76 d |
| Plu03 + HA | 6.49 d | 1.88 b | 996 c | 299.27 d |
| Plu03 + Az | 6.48 d | 1.66 cd | 850 d | 300.83 d |
| Plu03 + FA | 6.46 d | 1.83 b | 970 c | 288.14 d |
| SEMIA 4077 + Az | 6.29 d | 1.63 cd | 652 f | 276.87 d |
| SEMIA 4077 + HA | 6.18 d | 1.61 cd | 876 d | 252.19e |
| SEMIA 4077 + FA | 6.17 d | 1.57 cd | 854 d | 247.55 ef |
| N+HA | 5.64 e | 1.66 cd | 0 h | 226.04 fg |
| Plu03 | 5.60 e | 1.40 e | 724 ef | 225.98 efg |
| N + FA | 5.39 e | 1.56 d | 0 h | 211.92 g |
| SEMIA 4077 | 4.52 f | 1.26 f | 570 g | 174.15 h |
| Az | 1.69 g | 0.81 g | 0 h | 19.70 i |
| Control-N | 1.84 g | 0.78 g | 0 h | 15.47 i |
N: nitrogen; SDM: shoot dry mass; RDM: root dry mass; MDN: mass of dry nodules; NAC: nitrogen accumulated in the shoot. *Equal letters in the same column do not differ statistically (P<0.05) according to the Tukey test (n=5).
The potential benefits of the combined effects of rhizobia and A. brasilense co-inoculation and HS on the growth of lima bean plants were evidenced. The bioactivity of the application of HS to promote plant growth has been reported, such as the stimulation of photosynthesis by mechanisms similar to phytohormones, proliferation and elongation of roots due to H+-ATPase activity that induces cell division (Nardi et al., 2005; Dobbss et al., 2010; Trevisan et al., 2010; Canellas et al., 2011; Trevisan et al., 2011) and the assimilation of nutrients thanks to the presence of hydroxyl (OH) in carboxylic and phenolic groups (Muscolo et al., 2013). In a study conducted with faba bean plants (Vicia faba) treated with commercial HA applied on the leaves, increases in plant growth were observed (El-ghamry et al., 2009), In the common bean, stimulation of HA and FA on peroxidase activity has been reported to stimulate the growth and development of leaves. In common bean plants, the benefits of co-inoculation of rhizobia and Azospirillum in increasing crop growth and productivity have also been reported (Burdman et al., 1997; Hungria et al., 2013), while in lima beans, increases in SDM were observed in this study (Tab. 3).
Similar results of combined application of HS in conjunction with PGPR were observed in the common bean (Phaseolus vulgaris), where HA extracted from vermicompost in co-inoculation with Rhizobium tropici BR322, BR520 and BR534 strains and H. seropedicae strain HRC 54 in P. vulgaris plants cv. Grafite and cv. Bonus (originated from Brazil and Mozambique) submitted to water deficit, showed increases in SDM of plants both submitted or not to water stress compared to the non-inoculated control treatment that received nitrogen fertilization (Melo et al., 2017).
In the root dry mass (RDM) of lima bean plants (Tab. 3), the highest values were observed in plants co-inoculated with rhizobia and A. brasilense using the rhizobia isolate Plu14 and Plu03 in combined application with HA and FA (treatments Plu14+Az+HA and Plu14+Az+FA), followed by treatments SEMIA 4077+Az+HA and SEMIA 4077+Az+FA. The highest increases were in plants co-inoculated with rhizobia and A. brasilense (Plu03+Az; Plu14+Az; SEMIA4077+Az) and plants inoculated with rhizobia and HS (Plu03+HA; Plu03+FA; Plu14+HA; Plu14+FA; SEMIA4077+HA; SEMIA 4077+FA). These treatments showed no differences between them, but differed compared with the isolated inoculation of rhizobia and the control+N treatment. In addition, increases were observed in non-inoculated treatments that received N with HS (N+HA and N+FA) compared with control+N and isolated inoculation of rhizobia.
These results showed that co-inoculation of the rhizobia isolates (Plu14 and Plu03) and A. brasilense in conjunction with HS obtained higher increases in RDM in lima bean plants. This bioactivity of HS that induces elongation and proliferation of roots that modify the architecture of the root system by increasing H+-ATPase activity has been reported (Chen and Avaid, 1990; Façanha et al., 2002; Nardi et al., 2005; Zandonadi et al., 2007; Dobbss et al., 2010; Trevisan et al., 2010; Canellas et al., 2011; Canellas and Olivares, 2014; Canellas et al., 2015). Similar effects were evidenced in plants of the same family of fava beans (P. lunatus) as common bean (P. vulgaris) (Aydin et al., 2012; Melo et al., 2017). Similar results of co-inoculation of rhizobia and A. brasilense have been reported in common beans (Burdman et al., 1997; Hungria et al., 2013) and the combined application of HA and PGPR employing HA extracted from vermicompost in co-inoculation with the strains of Rhizobium tropici BR322, BR520 and BR534 and H. seropedicae strain HRC 54 in plants of Phaseolus vulgaris cv. Grafite and cv. Bonus subjected to water deficit. Significant increases were also evidenced in RDM both submitted or not to water stress compared to the non-inoculated control treatment that received nitrogen fertilization (Melo et al., 2017).
Regarding the mass of dry nodules (MDN) in lima bean plants, the greatest increases in MDN were seen in the treatments plu14+Az+HA and Plu14+Az+FA, followed by treatments Plu14+HA; Plu14+FA; Plu03+Az+HA and Plu03+Az+FA compared with the treatments inoculated only with the rhizobia isolates (Plu14 and Plu03). The greatest increases were seen in both the treatments of co-inoculation rhizobia and A. brasilense (treatments Plu03+Az; Plu14+Az and SEMIA 4077+Az) and the inoculation of rhizobia with HS (treatments Plu03+HA; Plu03+FA; Plu14+HA; Plu14+FA; SEMIA 4077+HA and SEMIA 4077+FA) compared to isolated inoculation of rhizobia.
These results show the potential of both the combined application of HS in co-inoculation with rhizobia and A. brasilense and the application of rhizobia with HS in increasing MDN in lima bean plants. There are reports on the bioactivity of HS in legumes such as soybean obtaining an increase in the number of nodules (Tilba and Sinegovskaya, 2012) and efficacy in triggering the expression of nod genes in mung bean plants (Vigna radiata) (Ahmad et al., 2012). The influence of co-inoculation of rhizobia and A. brasilense on nodules in common bean has been reported (Hungria et al., 2013).
Regarding the combined use of HS and PGPR, similar reports in soybean showed that inoculation with rhizobia in conjunction with sodium humate and ammonium molybdate in field increases significantly the number of nodules (Tilba and Sinegovskaya, 2012). However, in a study in common bean plants where Rhizobium tropici BR322, BR520 and BR534 and H. seropedicae HRC 54 strains in conjunction with HA (extracted from vermicompost) were co-inoculated, there were no increases in MDN (Melo et al., 2017), contrasting with what was evidenced in soybean plants and in the experiment with lima bean (Tab. 3).
In non-inoculated treatments that received nitrogen fertilization, with or without joint addition of HS (control+N; N+HA; N+FA) and N-Control, no nodules were observed, which ensured that the experiment did not cross-contaminate with rhizobia used in the other treatments.
The highest accumulation of N (Nac) in lima bean plants (Tab. 3) and higher relative efficiency (REI) of BNF (Fig. 1) was evidenced in plants that were co-inoculated with rhizobia and A. brasilense in combined application with HS (Plu14+Az+HA; Plu14+Az+FA; Plu03+Az+HA; Plu03+Az+FA; SEMIA 4077+Az+HA; SEMIA 4077+Az+FA), with higher values when HA was employed. In addition, increases in Nac and REI of plants were observed both in co-inoculation of rhizobia and A. brasilense and in the inoculation of rhizobia in combined application with HS.
These results suggest that the co-inoculation of rhizobia and A. brasilense in combined application with HS exert greater potential in the accumulation of N in the shoot of lima bean plants. The low amount of N contained in the HS applied would not be the cause of the increase of N of the plants. Similar studies of rhizobia inoculation have reported contributions in the accumulation of N in lima bean plants by BNF (Ormeño et al., 2007; Santos et al., 2009; Antunes et al., 2011; Santos et al., 2011; Duran et al., 2014; Servín-Garcidueñas et al., 2014; Ormeño-Orrillo et al., 2017).
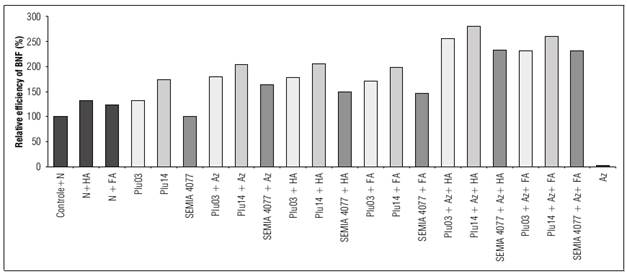
Figure 1 Relative efficiency index of biological nitrogen fixation by inoculation or co-inoculation of rhizobia (Plu03, Plu14 and SEMIA 4077) and A. brasilense (Az) combined with humic substances (humic acids [HA] and fulvic acids [FA]) in lima bean plants.
Regarding the co-inoculation of rhizobia and Azospirillum, some studies with legumes such as common bean, soybean (Hungria et al., 2013) and lentils (Kumar and Chandra, 2008) reported increases in Nac in plants. Similarly, the effects of HS have been reported as modulators of metabolic processes in plants, such as increased H+-ATPase activity, alteration of nitrogen metabolism, and photosynthesis (Canellas et al., 2013).
Similar results of combined application of HS in co-inoculation of rhizobia with A. brasilense obtained in this study in Nac in lima bean plants (Tab. 3) were observed in soybean, where inoculation with rhizobia in the presence of sodium humate (humic acids with sodium) and ammonium molybdate in the field significantly increased the Nac in the plants (Tilba and Sinegovskaya, 2012).
Non-inoculated treatments that received N with HS (N+HA; N+FA) increased Nac in plants when compared to the control treatment with N (control+N) (Fig. 1). The contribution of HS in the accumulation of N in plants may have occurred by inducing HS in the modulation of metabolic processes such as N absorption through membrane permeability facilitated by H+-ATPase activity (Canellas et al., 2013).
Effect of the combined application of HS and co-inoculation of rhizobia and A. brasilense in leucaena plants
The results of the variables evaluated in the growth promotion of leucaena plants in response to the treatment with rhizobia inoculated or co-inoculated with A. brasilense in conjunction with HS are presented in table 4.
Table 4. Effect of inoculation and co-inoculation of rhizobia (Leu01, SEMIA 4081, SEMIA 6361) and A. brasilense (Az) combined with humic acids (HA) and fulvic acids (FA) in the promotion of growth of leucaena plants.
| Treatments | SDM | RDM | NN | Nac |
|---|---|---|---|---|
| (g) | (mg) | |||
| Control+N | 1.61 l | 0.22 f | 0 f | 29.35 h |
| N + HA | 2.00 ij | 0.44 cd | 0 f | 42.55 f |
| N + FA | 1.94 jk | 0.37 de | 0 f | 40.52f |
| Leu01 + Az+ HA | 3.00 a | 0.63 ab | 57 a | 73.13 ab |
| SEMIA 6361 + Az+ HA | 2.93 ab | 0.67 ab | 67 a | 74.93 a |
| Leu01 + Az+ FA | 2.92 ab | 0.60 b | 56 a | 69.82 bc |
| SEMIA 4081 + Az+ HA | 2.81 bc | 0.69 a | 56 a | 71.02 ab |
| SEMIA 6361 + Az+ FA | 2.73 cd | 0.61 ab | 61 a | 66.48 c |
| SEMIA 4081 + Az+ FA | 2.61 d | 0.64 ab | 59 a | 61.84 d |
| Leu01 + FA | 2.37 e | 0.42 cde | 42 bc | 51.48 e |
| Leu01 + Az | 2.36 ef | 0.46 c | 42 bc | 51.59 e |
| Leu01 + HA | 2.35 ef | 0.46 c | 46 b | 51.93 e |
| SEMIA 4081 + Az | 2.27 efg | 0.45 c | 40 c | 52.59 e |
| SEMIA 4081 + HA | 2.23 fgh | 0.42 cde | 43 bc | 50.48 e |
| SEMIA 6361 + Az | 2.18 gh | 0.44 cd | 40 bc | 48.99 e |
| SEMIA 6361 + HA | 2.14 hi | 0.44 cd | 41 bc | 48.68 e |
| SEMIA 4081 + FA | 1.91 jk | 0.37 de | 38 c | 43.06 f |
| SEMIA 6361 + FA | 1.86 k | 0.35 e | 41 bc | 40.37 f |
| Leu01 | 1.67 l | 0.26 f | 28 d | 35.44 g |
| SEMIA 4081 | 1.63 l | 0.25 f | 24 d | 35.42 g |
| SEMIA 6361 | 1.58 l | 0.25 f | 27 d | 31.10 h |
| Az | 0.31 m | 0.07 g | 0 f | 0.44 i |
| Controle-N | 0.31 m | 0.06 g | 0 f | 0.43 i |
N: nitrogen; SDM: shoots dry mass; RDM: root dry mass; NN: number of nodules; NAC: nitrogen accumulated in the aerial part. *Equal letters in the same column do not differ statistically (P<0.05) according to the Tukey test (n=5).
This experiment showed the that the greatest increase in SDM in leucaena plants occurred when they were co-inoculated with rhizobia and A. brasilense in combined application with HS (treatments Leu01+Az+HA; Leu01+Az+FA; SEMIA 4081+Az+HA; SEMIA 4081+Az+FA; SEMIA 6361+Az+HA and SEMIA 6361+Az+FA), followed by inoculation of rhizobia with HS (treatments Plu03+HA; Plu03+FA; Plu14+HA; Plu14+FA; SEMIA 4077+HA and SEMIA 4077+FA) and co-inoculation rhizobia and A. brasilense (Leu01+Az; SEMIA 4081+Az; SEMIA 6361+Az), and finally, the addition of N with HS (N+HA; N+FA), when compared to control+N (Tab. 4).
These results showed the beneficial effects of the combination of rhizobia and A. brasilense co-inoculation and the bioactivity of HS in the growth of leucaena plants, which can be explained by the benefits of the application of both HS and PGPR that have been reported in several studies. Regarding the effects of HS, there are reports that show that they promote plant growth by stimulating photosynthesis by mechanisms similar to phytohormones, root proliferation and elongation due to H+-ATPase activity that induces cell division (Nardi et al., 2005; Trevisan et al., 2010; Dobbss et al., 2010; Canellas et al., 2011; Trevisan et al., 2011), and the assimilation of nutrients due to the presence of OH in the carboxylic and phenolic groups (Muscolo et al., 2013).
In leucaena plants, it was reported that the application of HA extracted from lignite contributed to the increase of SDM of plants (Murgas and Falla, 2016). As well, some studies showed the efficiency of rhizobia in the BNF and benefits in promoting growth in leucaena plants (Bueno and Camargo, 2015; Aguirre-Medina et al., 2015). The results of this experiment (Tab. 4) also showed increases in SDM of leucaena plants by co-inoculation of rhizobia and A. brasilense, when compared with the isolated inoculation of rhizobia and control+N, similar to the increases reported in studies in soybean and common bean plants co-inoculated with rhizobia and A. brasilense (Hungria et al., 2013).
In relation to the combined application of HS and PGPR, similar results have been reported in other legumes. In the case of common bean (P. vulgaris), HA extracted from vermicompost were tested in co-inoculation with PGPR in P. vulgaris plants submitted to water deficit. Increases were evidenced in SDM of plants both submitted or not to water stress compared to the non-inoculated control treatment that received nitrogen fertilization (Melo et al., 2017). In soybean, sodium humate was applied in conjunction with rhizobia in the seed, showing increases in plant growth compared to the control (Tilba and Sinegovskaya, 2012).
Regarding the dry mass of the root (RDM) of leucaena plants, the highest increases were observed in the treatments co-inoculated with rhizobia and A. brasilense in combined application with HS (HA and FA) (Leu01+Az+HA; Leu01+Az+FA; SEMIA 4081+Az+HA; SEMIA 4081+Az+FA; SEMIA 6361+Az+HA; SEMIA 6361+Az+FA), followed by treatments co-inoculated with rhizobia and A. brasilense, treatments inoculated with rhizobia with HS (HA and FA), and treatments that received N with SH (N+HA and N+FA); compared with the non-inoculated control treatment that received N (control+N) and treatments inoculated alone with rhizobia.
These results showed the beneficial effects exerted by the co-inoculation of rhizobia and A. brasilense in combined application with HS (HA and FA) on the growth of the roots of leucaena plants. The bioactivity of HS has been reported to induce the elongation and proliferation of roots that modify the root system architecture by increasing H+-ATPase activity which induces elongation and cell division (Chen and Avaid, 1990; Façanha et al., 2002; Nardi et al., 2005; Zandonadi et al., 2007; Dobbss et al., 2010; Trevisan et al., 2010; Canellas et al., 2011; Canellas and Olivares, 2014; Canellas et al., 2015). Similar studies of combined application of HS and PGPR have been reported in legumes such as common bean (P. vulgaris), where HA were tested in co-inoculation with Rhizobium tropici BR322, BR520 and BR534 strains and H. seropedicae strain HRC 54, showing increases in RDM both in plants submitted or not to water stress (Melo et al., 2017).
In leucaena plants, the highest number of nodules (NN) was observed in plants that were co-inoculated with rhizobia and A. brasilense in combined application with HS (Leu01+Az+HA; Leu01+Az+FA; SEMIA 4081+Az+HA; SEMIA 4081+Az+FA; SEMIA 6361+Az+HA; SEMIA 6361+Az+FA), followed by plants that were co-inoculated with rhizobia and A. brasilense (Leu01+Az; SEMIA 4081+Az; SEMIA 6361+Az) and plants inoculated with rhizobia in combined application with HS (Leu01+HA; Leu01+FA; SEMIA 4081+HA; SEMIA 4081+FA; SEMIA 6361+HA; SEMIA 6361+FA), compared with plants inoculated with rhizobia alone.
Similar studies in legumes such as soybeans where only HA were applied reported increases in the number of nodules (Tilba and Sinegovskaya, 2012) and in V. radiata, the efficacy of HA in triggering nod gene expression (Ahmad et al., 2012). In relation to the co-inoculation of rhizobia and A. brasilense in leucaena plants (Tab. 4), the results showed increases in the number of nodules, similar to that reported in some studies in common bean, soybean (Hungria et al., 2013), and lentils (Kumar and Chandra, 2008). In addition, similar results obtained by inoculation or co-inoculation of PGPR in the presence of HS were reported in soybean (Tilba and Sinegovskaya, 2012). On the other hand, the results obtained in this study (Tab. 4) differ from those found in common beans, where there was no evidence of increases in NN by co-inoculation of rhizobia and H. seropedicae (Melo et al., 2017).
The results of the accumulated nitrogen (Nac) determined in the shoot of the leucaena plants showed that the highest accumulation of N (Tab. 4) was reflected in the highest relative efficiency index (REI) of the BNF (Fig. 2) in the plants that were co-inoculated with rhizobia and A. brasilense in combination with HS (treatments Plu14+Az+HA; Plu14+Az+FA; Plu03+Az+HA; Plu03+Az+FA; SEMIA 4077+Az+HA and SEMIA 4077+Az+FA). This was followed by plants that were co-inoculated with rhizobia and A. brasilense, then by plants inoculated with rhizobia with HS, which increased Nac and REI (there were no differences between these treatments), compared with plants inoculated with rhizobia alone and the uninoculated control that received N (control+N).
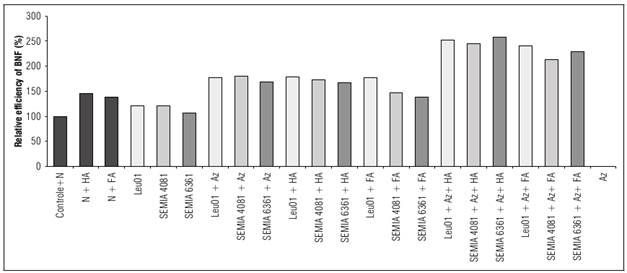
Figure 2. Relative efficiency index of biological nitrogen fixation by inoculation or co-inoculation of rhizobia and A. brasilense in conjunction with humic substances in leucaena plants.
These results showed the efficiency of combining the effects of co-inoculation of rhizobia and A. brasilense with HS, which contributed to the greatest stimulation of Nac in the shoots of leucaena plants and higher relative efficiency of BNF. Regarding these effects, there are reports of the contributions of isolated rhizobia inoculation in Nac and REI of BNF in leucaena plants (Aguirre-Medina et al., 2015; Bueno and Camargo, 2015). There are also reports that show the effects of HS as modulators of metabolic processes in plants, such as increased H+-ATPase activity, alteration of nitrogen metabolism, and photosynthesis (Canellas et al., 2013). Similar results in the increase of Nac and REI of BNF by the combined application of HS in co-inoculation with rhizobia have been reported in soybean crops. In these crops, significant Nac increases were observed when inoculated with rhizobia in the presence of sodium humate (humic acids with sodium) and ammonium molybdate in the field (Tilba and Sinegovskaya, 2012).
The results obtained in these two experiments in lima bean and leucaena plants showed that the co-inoculation of rhizobia and A. brasilense in combined application with HS could be considered a potential tool in the promotion of plant growth, not only as an economic alternative but as a sustainability strategy.
CONCLUSION
The co-inoculation of rhizobia and A. brasilense in combined application with humic substances (humic acids and fulvic acids) promotes greater growth of fava bean plants, stimulating an increase in shoot dry mass, root dry mass, nitrogen accumulation in the shoot and the relative efficiency index of biological nitrogen fixation of the plants. This highlights the significance of the inoculation of the rhizobia isolate Plu14 and the application of HA.
The growth promotion of leucaena plants is maximized when the plants are co-inoculated with rhizobia and A. brasilense in conjunction with humic substances (humic acids and fulvic acids). This increases shoot dry mass, root dry mass, and nitrogen accumulation in the shoot, as evidenced by the enhanced relative efficiency index of biological nitrogen fixation.















