Introduction
Worldwide, prostate cancer is the fourth-most diagnosed neoplasm and the second-leading cause of cancer death in men. Countries with lower Human Development Index have higher mortality rates, and prostate cancer becomes the leading cause of death1. In these countries, timely access to early detection, diagnosis, and curative treatments, such as radiation therapy, is lacking. Radiation therapy can be curative in most patients with newly diagnosed localized prostate cancer or in the salvage setting after radical prostatectomy2,3. It also has the potential to elicit durable responses in selected patients with low-volume metastatic disease4. Accurate characterization of the extent of the disease improves the success of radiation therapy in all settings.
The development and implementation of more precise imaging methods, such as prostate-specific membrane antigen (PSMA) positron emission tomography (PET), have resulted in higher sensitivity compared to conventional imaging methods (CIM) for staging in patients with prostate cancer. In the Pro PSMA study, PSMA-PET showed 27% better detection for all disease sites in unfavorable intermediaterisk and highrisk localized cancer prior to curative intent surgery or radiotherapy5. However, better imaging methods in oncology may offer benefits in patient staging that may not translate entirely into improved oncologic outcomes6. Based on clinical trial data, at least 15-20% of men with high-risk prostate cancer will develop metastatic disease in long-term follow-up7. The hypothesis is that a preexisting disease was not revealed by CIM but was partly detected by PSMA PET. The question remains how PSMA PET and detection of this previously undetected disease may affect cancer treatment, especially in patients with nodal involvement and metastatic disease, in which PSMA PET/CT may alter and change the treatment plan. In theory, better imaging and, therefore, better staging would result in better guidance of oncologic treatment. This can influence how we approach patients with a curative vs. palliative intent, with the risk of undertreatment for some patients who would not be palliative on CIM. The role of PSMA PET in guiding radiation therapy treatment plans is currently being established. In a recently published systematic review, PSMA PET resulted in nearly a 50% change in initial radiation therapy treatment planning, both in postradical prostatectomy and prior to salvage radiation therapy8. This is critical in some scenarios, such as salvage radiation therapy, where the extent of the target volume will define oncologic outcomes, mainly with regard to suspected nodal involvement. New evidence has shown that 69% of lymph node metastases occur outside of the target volumes of traditional contouring guidelines; the site of disease revealed by PSMA PET can be used to validate new contouring lymph node atlases to guide treatment planning9.
Colombia has pioneered the regional development of PSMA PET/CT in Latin America. The technology has been available since 2016 and is rapidly becoming more available for prostate cancer patients. Here, we carried out a retrospective study with patients referred to radiation therapy based on a PSMA PET/CT study in both an intact naïve treatment setting and a postoperative biochemically recurrent setting. We sought to establish the role of PSMA PET in the decision-making and change of treatment plans in a Latin American country cohort.
Methods
Patients and outcomes
We conducted a retrospective observational study of patients referred to a private radiation oncology practice specializing in genitourinary malignancies in Bogota, Colombia, from January 2020 to April 2021, with available 68Ga-PSMA PET/CT or 18F-PSMA PET/CT reports at the moment of decision-making regarding radiation therapy treatment. An independent local private IRB approved the study protocol in January 2021. Our primary outcome was to establish the role of PET-PSMA in decision-making regarding radiation therapy treatment plans, which was defined as changes in target volume in comparison to CIM based on PET-PSMA results before radiation therapy. Eligible patients were those with biopsy confirmation of prostate cancer. The included patients could be initially treated with curative-intended radical prostatectomy and referred for PET-CT assessment at biochemical recurrence or persistence (defined as PSA levels > 0.2) or patients referred for radiation therapy as primary treatment. For comparison, all patients had conventional imaging results from CT or MRI scans of the abdomen/pelvis or bone scintigraphy. Patients were deemed not eligible if the treatment planning included a previously treated radiation therapy field. As a secondary outcome, we aimed to establish the role of PET PSMA in restaging patients, defined by the rate of detection of the presence of distant metastases (bone and visceral), pelvic nodal involvement, and tumor bed that were not present on CIM. Changes in treatment intent were also considered (palliative vs curative intent).
Treatment
All treatment plans were delimited in Monaco HD (Elekta-v5.0) with a previous fusion of PET images in the treatment position. Radiation was administered using a Sinergy linear accelerator with daily kilo voltage imaging using volumetric modulated arc therapy (VMAT). Treatment plans for patients receiving VMAT were constructed and reviewed based on the NRG Oncology and Radiation Therapy Oncology Group Contouring Atlases (RTOG; Hall-Harris et al.). This study defined all treatment volume changes based on PET-PSMA scan results as changes from conventional treatment plans of the RTOG contouring guidelines. Based on National Comprehensive Cancer Network and American Society for Radiation Oncology guidelines, treatment doses were selected according to each patient's characteristics.
Statistical analysis
We collected the clinical information from a previously established database. Data were used to form a general description of the study population. Subsequently, the results obtained from conventional images and PSMA PET were analyzed, correlating them with disease management. Finally, the impact on radiotherapy use is described in reference to the total accumulated dose and the included irradiation fields. For the statistical analysis, the variables were standardized. We utilized central tendency and dispersion measures for continuous variables; the median and percentile-type dispersion measures were used whenever there were no values with normal distribution. For categorical variables, percentages and proportion measures were used.
Results
Patients' demographics
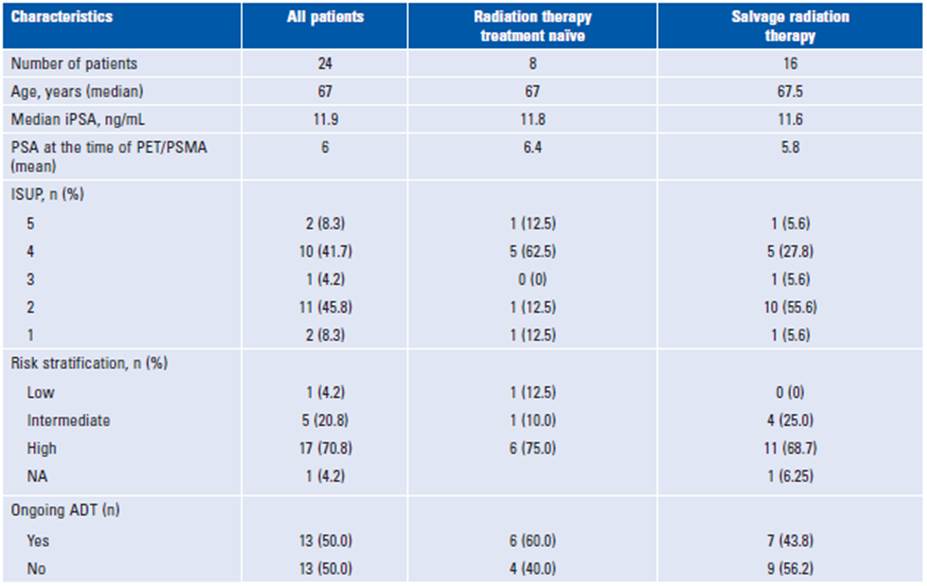
Between January 2020 and April 2021, 26 patients were referred to the radiation oncology practice with a PSMA PET/computed tomography (CT) result available. Two patients had previously received prostate radiation therapy and were deemed ineligible for analysis. A total of eight patients were treatment naïve, and 16 patients had a postoperative biochemical recurrence and were referred for salvage radiation therapy. The patient's baseline characteristics are summarized in Table 1. Overall, 17 patients had the high-risk disease at diagnosis, according to the D'Amico classification. Primary management and type of surgery (open vs robotic) varied among the patients referred for salvage radiation therapy, and four patients did not undergo lymph node dissection.
Conventional imaging
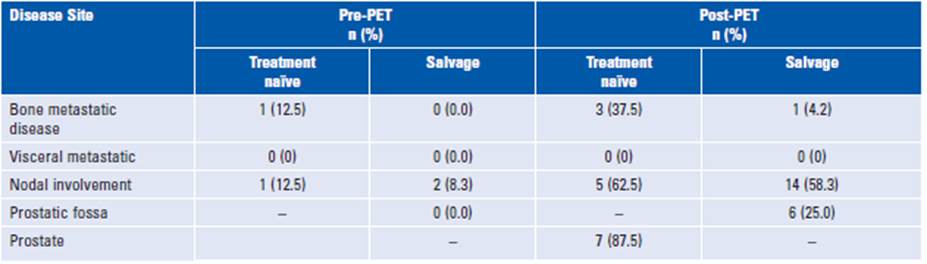
For comparison, conventional imaging (CT, MRI, or bone scan) was available for all patients. Only 17% of patients had positive findings based on CIM, whereas 89% had positive findings based on PSMA PET/CT. Different anatomic involvement sites are described in Table 2. With conventional imaging, bone metastases were diagnosed in two out of eight of the treatment-naïve patients and none of the salvage radiation therapy group. PSMA PET/CT detected bone involvement in 30.0 and 6.2% of patients in the former and latter groups, respectively. Overall, PSMA PET/CT improved detection by 77% in curative radiotherapy and 69% in patients undergoing salvage radiation therapy compared to conventional imaging.
Lymph node involvement
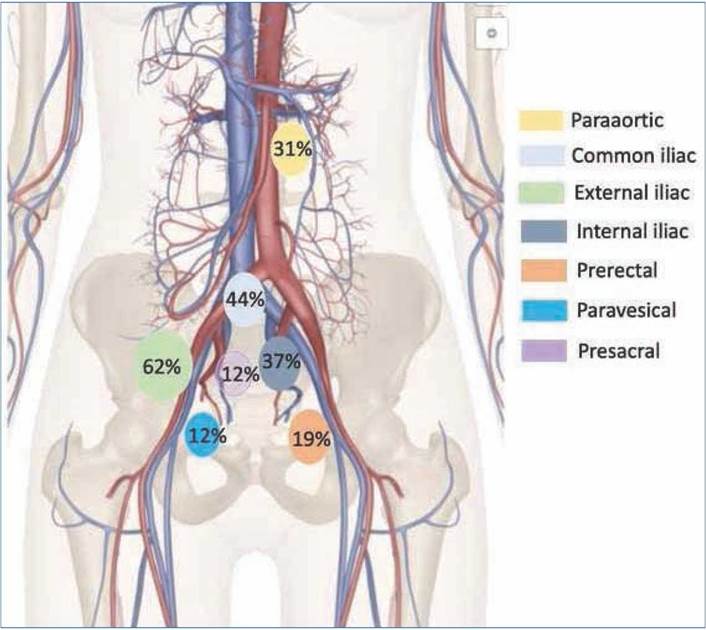
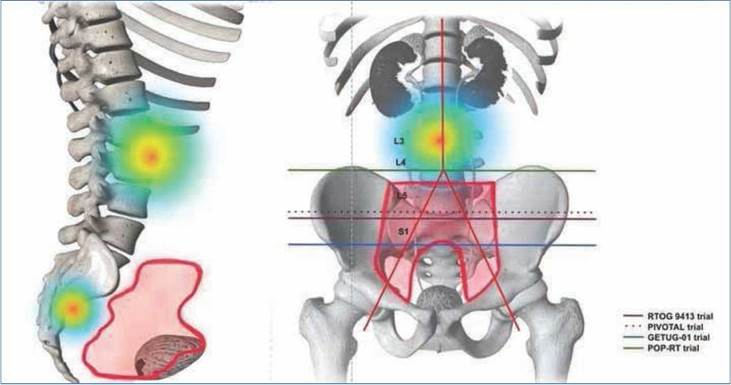
Lymph node metastases are shown in Figure 1 based on the involved nodal chain. Of the patients, 26.8% had lymph node involvement outside the traditional pelvic lymph node volume definitions: paraaortic nodes 11.5% (n = 3), perirectal nodes 11.5% (n = 3), and paravesical nodes 3.8% (n = 1). PSMA PET-positive lymph nodes that were not included in the GETUG 01/RTOG 9413/POP-RT/ PIVOTAL protocols were para-aortic and perirectal lymph nodes (Fig. 2)10.
Changes in the treatment plan
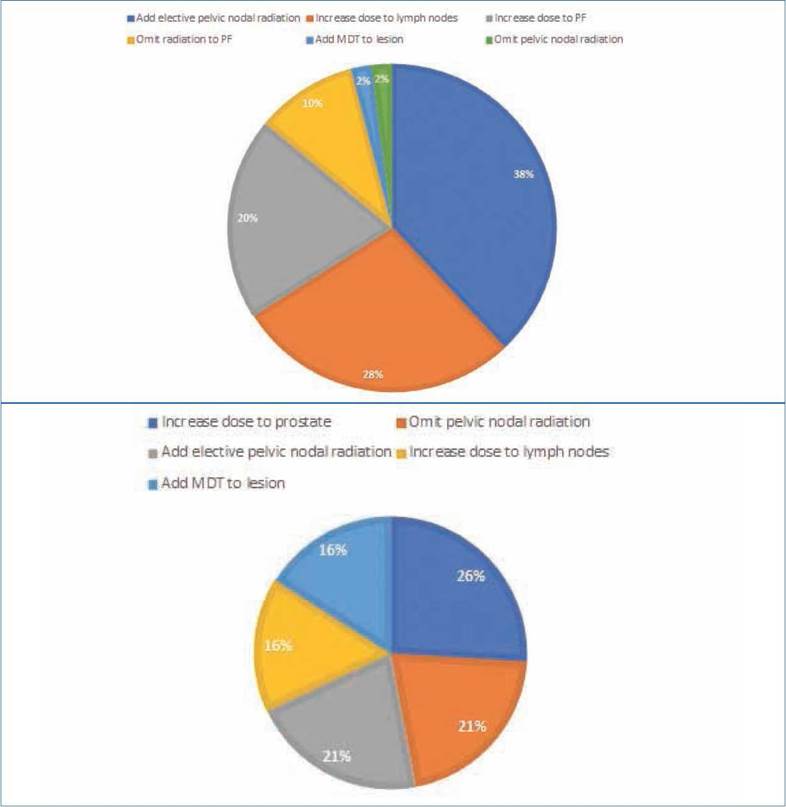
Based on a recently published review on the use of PET/PSMA for radiation therapy treatment plans, we categorized treatment changes depending on the location of the disease detected by PET imaging and its influence on the radiation dose and radiation volume8. Treatment considerations following PET imaging are presented in Figures 3A and B. For the salvage patients, we divided target volume changes in regard to the inclusion or exclusion of prostate fossa lesions, pelvic nodal lesions, or distant metastatic lesions. For the treatment-naïve patients, it was the prostate instead of the prostatic fossa. Most treatment changes were observed in the salvage setting as the inclusion of elective pelvic nodal radiation, followed by an increased dose to the lymph nodes and the prostate fossa. In the treatment-naïve patients, most changes were related to an increase in the dose to the prostate, followed by the addition or omission of pelvic nodal radiation.
Discussion
Our study showed that the use of PSMA PET/CT impacted the decision-making process in radiation treatment planning for our patients, both in the salvage setting and before definitive radiation therapy, by improving disease detection over CIM. We had a 72% higher disease detection rate than CIM (89 vs 17%). In the treatment-naïve patients, this meant a staging change in 75% of the patients, which is comparable to results shown in other retrospective and prospective studies of PSMA, which ranged from 21.0 to 50.8%5,11,12. In the salvage setting, the more prominent component of our series, this meant restaging in 50% of the patients. Overall, we obtained higher results than other series, which could be related to differences in the group risks of the included patients and the interobserver variability and quality of the imaging methods used for comparison. Management radiation therapy changes based on PSMA PET/CT can be thought of in terms of escalation or deescalation of treatment, which can be due to alteration in the radiation dose or target volumes.8 Prior retrospective studies have reported management changes in up to 30.2-52.0% of patients13,14, with growing phase3 evidence supporting changes in the treatment approach based on PET-PSMA for up to 56.8% of patients who had undergone primary definitive treatment and presented with increasing PSA levels15. This is mainly due to the detection of diseases outside traditional CTV. In our series, the main changes were related to the higher detection of bone metastases and node involvement. Elective nodal radiation is still controversial in prostate cancer management, and it is currently based on nomograms developed before the use of molecular imaging16. In the salvage setting, we safely omitted the pelvic radiation in one patient, with most of our patients (62.5%, n = 15) having pelvic lymph nodes included as part of their radiation treatment due to the positive findings on PET/PSMA.
Although we did not report toxicity outcomes, we know from the RTOG 0534 SPPORT trial (a randomized trial) that with the use of Intensity Modulated Radiation Therapy (IMRT), patients present with similar toxicity profiles to those resulting from tumor bed radiation17. This has also been validated in population-based studies on patients treated with upfront radiation therapy18. One of our most relevant treatment changes was the inclusion and boosted doses of radiation to involved nodes outside the traditionally defined target nodal volumes. PSMA PET imaging data have shown that historical RTOG contouring atlases can disregard lymph node portions where recurrent nodal disease dwells. In a retrospective analysis by C Onal et al.19, lymph nodes outside the pelvic fields were as high as 30.4% (para-aortic and presacral lymph nodes). Our results were higher, with 40% of lymph nodes outside the pelvic fields defined by GETUG-01 and RTOG20,21. In our series, 18 patients received a SIB to clinically positive lymph nodes to recommended doses higher than 60 Gy for clinically positive lymph nodes, which have better oncological outcomes22. Four of our patients had metastasis-directed therapy they would not have received based on CIM. In a retrospective cohort of Italian patients, when compared to 18F choline PET, PSMA PET-guided stereotactic body radiation therapy resulted in higher rates of androgen deprivation therapy (ADT) free survival in patients with oligo-recurrent castration-sensitive prostate cancer23. Furthermore, two prospective clinical trials (STOMP and ORIOLE) have shown that metastasis-directed therapy can impact ADT-free survival for patients with oligometastatic disease24,25. However, both trials used PET choline and CT imaging, with the ORIOLE trial PSMA PET results blinded to the investigators. ORIOLE has shown promising early results in patients with consolidation with PSMA results. However, a longer follow-up period is needed to obtain the final predicted outcomes.
In general, PSMA PET/CT allowed us to include more target-directed volumes of disease not previously evident on CIM. The question remains how PSMA PET/CT impacts oncological outcomes by upstaging or downstaging patients and altering treatment volumes. The degree to which higher accuracy can impact radiation therapy treatment planning is currently under study in randomized controlled trials26,27. It is hypothesized that superior accuracy can ultimately have a management impact since radiation therapy usually benefits from a more accurate depiction of the anatomic distribution of disease. Until oncological outcomes of ongoing prospective studies on PET-PSMA changes in treatment plans become available, new nomograms based on the prognostic value of PSMA PET/CT data are being developed. M Xiang et al. proved that this nomogram could be superior in terms of risk stratification to CAPRA, STARCAP, and the preprostatectomy nomogram of the MSKCC. This provides valuable data, as it predicts that the risk of upstaging with PSMA PET is related to long-term clinical outcomes, such as metastasis and cáncer specific mortality, meaning that PSMA PET can be viewed as a prognostic biomarker in prostate cancer28.
To the best of our knowledge, this is the first study describing the effect of PSMA PET/CT on the alteration of radiotherapy planning in a Colombian cohort. Nonetheless, it has limitations, mostly related to its retrospective nature. This can only be overcome by prospective randomized trials; however, PSMA availability in the country is still limited. The small number of patients might be explained by Colombia's healthcare system, in which there is an approval process that may delay access to this technology. A costeffective analysis adapted to our health system is needed to encourage insurance companies to increase the availability of PSMA PET/CT and ensure prompt access for prostate cancer patients in the country29.
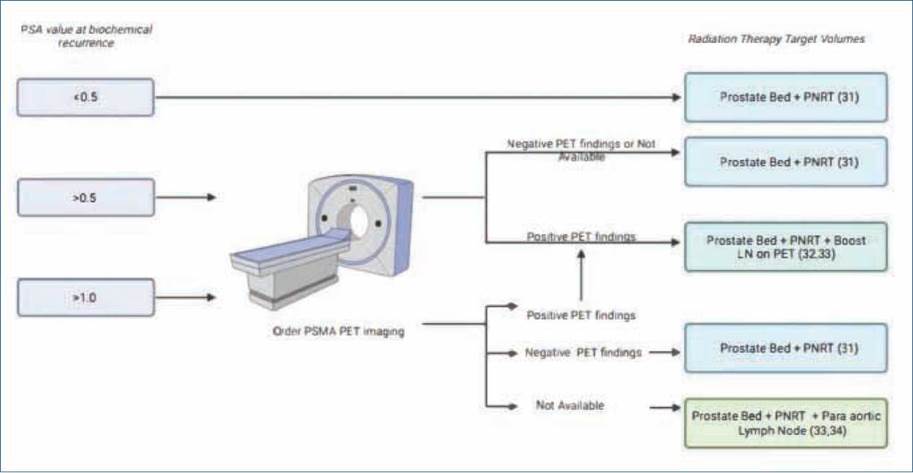
Not all PET/PSMA used the same radiotracer or were performed at the same institution, but this technology is only available in a few centers, and health insurance agencies (EPS, from the Spanish Entidades Promotoras de Salud) have the power to authorize its use at the preferred site30. Whether this detection technique has an impact on oncological outcomes remains to be tested. Subsequently, an analysis of its cost-effectiveness should be undertaken, especially in countries with low to middleincome economies, such as Colombia. Columbia has universal health coverage, which means that this compulsory insurance covers all diagnostic and treatment costs30. According to the current exchange rate (US $1 = 3897.00 Colombian pesos), the authors note that a PSMA PET/CT fee can range from 1,539 to 2,051 USD. The introduction of this new technology must be balanced considering the benefits in oncological outcomes, which is a pressing issue in countries with bad economies, as it can impose an additional burden on the health system. Timely referrals for radiation oncology may vary among institutions and countries due to differences in healthcare payment systems. In our case, in Colombia, a timely referral may be hindered by access to radiation therapy machines, and we believe it will not be delayed by access to PSMA/PET, construction of new contouring guidelines based on the additional knowledge provided by PSMA/PET regarding patterns of disease spread and recurrence is needed, with new management algorithms to be considered (Figure 4)31-33.
Conclusions
Our study is the first of its kind published in Colombia. PSMA PET/CT impacts the practice of radiation oncologists as it changes the traditional radiotherapy treatment fields that have been used for decades. This new information could explain the previous therapeutic failures derived from untreated micrometastatic disease. More precise images are the basis for the development of radiotherapy, with a potential increase in the therapeutic ratio of radiotherapy with each technological advance in diagnostic images.