Introduction
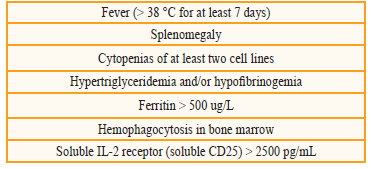
The hemophagocytic syndrome (HPS) is a rare clinical entity caused by increased activation of T lymphocytes, macrophages and histiocytes in liver, spleen and bone marrow that results in hypercytokinemia and alterations of the immune system[1,3]. It can be inherited or acquired[4]. Some secondary causes are immunodeficiencies[5], hematologic neoplasias, and infectious processes with different etiological agents[6]; Epstein Barr virus, cytomegalovirus and fungi, among others. The cardinal symptoms of HPS are: prolonged fever, hepatosplenomegaly, and pancytopenia. High levels of cytokines and ferritin lead to inhibition of erythropoiesis; activated macrophages increase levels of plasminogen activating factor, leading to hyperfibrinolysis, and organomegaly; neurological symptoms and cholestatic hepatitis can be attributed to infiltration by activated lymphocytes and histiocytes[3]. The diagnosis is made based on 5 criteria (Table 1) and treatment is that of the triggering factor[1].
Table 1 Diagnostic criteria for hemophagocytic syndrome (must meet > 5 for diagnosis). Fever (> 38 °C for at least 7 days)
Histoplasmosis is an opportunistic infection that may have multiple clinical manifestations, one of which is the disseminated form that, although infrequent, leads to a high mortality rate. Diagnosis is difficult because Histoplasma antigens can be falsely negative due to SHF and chronic immunosuppression, which usually require tissue samples whose results can take several days delaying the start of treatment and worsening prognosis[1].
Transplanted patients are particularly prone to opportunistic infections due to chronic immunosuppression. In Colombia, the incidence of disseminated Histoplasma capsulatum infection is low, close to 1.1%, but mortality rates are high, some series have described up to 80% of cases without treatment[2,7]. In one US cohort severe disease occurred in 38% of case (defined as admission to intensive care unit) and 81% had disseminated histoplasmosis[8]. In cases where these two entities coexist, mortality can approach 100% of cases without treatment[9].
Below is the case of a renal transplant elderly patient, who had acquired hemophagocytic syndrome, secondary to disseminated histoplasmosis and favorable evolution with antifungal treatment.
Presentation of the case
A 78-year-old woman with polycystic kidney disease who had a kidney transplanted 8 months ago from cadaveric donor of extended criteria, and received basiliximab induction therapy and then maintenance therapy with prednisolone, mycophe-nolate mofetil and tacrolimus XL (levels between 5 and 8 ng/mL). She went to the doctor because she was suffering malaise, fever, altered mental status, and cough. During physical examination, she was asthenic, adynamic, pale; her vital signs were blood pressure 100/56, heart rate 105 beats per minute, temperature 38.5 °C; and exhibited fine bibasilar crackles, splenomegaly, graft in the right iliac fossa, no pain on palpation and no skin lesions. Initial paraclinics documented pancytopenia, hypovolaemic hyponatremia, and azotize increase; chest x-ray reported interlobular septal thickening in right hemithorax and serum procalcitonin at 18.5 ng/mL (positive). Hydric resuscitation and antibiotic coverage with cefepime were initiated; however, she did not show improvement and continued with a systemic inflammatory response, so the antibiotic regimen was changed to meropenem; the cultures of blood and urine were negative, but the patient remained febrile and with pancytopenia. Therefore, studies were expanded: lactate dehydrogenase (LDH) 4680 IU/L, ferritin > 40,000 ug/L, triglycerides 544 mg/ dL (high), and fibrinogen 153 mg/dL (decreased) (Table 2), for which hemophagocytic syndrome, probably secondary, was diagnosed. We continued the search for the cause and the peripheral blood smear showed yeast from Histoplasma capsulatum (Figure 1). The diagnosis was subsequently confirmed with the positive Histoplasma urine antigen result at 121.7 ng/mL. Antifungal coverage with liposomal amphotericin B (1 mg/kg for 14 days) was initiated and a bone marrow biopsy was performed which showed blockage in neutrophil maturation, hemophagocytosis and histoplasmosis. In addition, viral load was detected by positive cytomegalovirus (CMV) PCR (polymerase chain reaction) in 18,000 copies, so that intravenous ganciclovir was added.
The patient showed favorable clinical evolution, with resolution of pancytopenia, improvement of neurological symptoms and stabilization of renal function. She was discharged and valganciclovir treatment was given until obtaining two viral loads for negative CMV, oral itraconazole 200 mg every 8 hours for 3 days and then 200 mg every 12 hours. After a 6-month follow-up Histoplasma urine antigen remained positive at 2.7 ng/mL. To date, she has completed 1 year of itraconazole treatment with adequate clinical response and clearance of urine antigen.
Discussion
Infections represent the second leading cause of death in long-term follow-up studies of ransplanted patients. They can be caused by viruses, bacteria, parasites and fungi. In Colombia, a series of patients with renal transplantation and histoplasmosis compromise with an incidence of 1.1% have been reported in Medellín, 2 of 9 cases occurred in the first year of transplantation, 88% required immunosuppression adjustment due to rejection prior to infection, and 88% had graft dysfunction with creatinine greater than 1.5 mg/dL3.
Histoplasmosis is the most frequent mycosis in the Américas[10], causing about 500,000 infections annually according to the CDC (Center for Disease Control) and being endemic in some countries. The etiologic agent is Histoplasma capsulatum. Moderate temperatures, the presence of bird or bat guano, activities such as agriculture, exposure to chicken coops or caves, remodeling or demolition of old buildings, and logging have been associated with contagion[10]. Infection is acquired by inhaling the spores and severity of the disease depends on the number of inhaled spores and the host's immune status; the majority of infections are asymptomatic[8], but there are cases in which there is involvement of more than two organs (lung, liver, spleen, bone marrow and central nervous system), called disseminated histoplasmosis, an entity that can have a mortality around 10% with treatment and up to 80% without timely treatment[8]. Infection can occur by primary infection, secondary infection in patients with previous exposure to large inoculums, and reactivation of latent infection. Transmission by the donor is rare, but there are some published case reports[8].
Dissemination outside the lung is common, but is rarely clinically recognized. Cellular immunity can control dissemination, but immunosuppressed and elderly patients, such as our patient, have a 10-fold increased risk of developing disseminated histoplasmosis[8,10]. The clinical picture presented in this case is similar to that described in the literature: nonspecific, with asthenia, adynamia, weight loss, anorexia, some respiratory symptoms (50% of cases) and, during physical examination, lymphadenopathy, hepatosplenomegaly (25-60% of cases) and, less frequently, oral or skin lesions can be found. The mean time from transplant to diagnosis was 27 months in a cohort of 152 patients, of which 34% were diagnosed in the first year after transplantation8, as in the present case.
Laboratory tests are also non-specific, may show anemia, leukopenia, thrombocytopenia, and elevated liver injury tests. Within evolution, dysfunction of multiple organs, adrenal insufficiency, and even hemophagocytic syndrome[11], as in this case[7,9], have been described. Central nervous system involvement (meningitis, cerebritis and focal lesions) and spinal cord involvement have been reported in 5-10% of cases. The findings in the patient's pulmonary imaging are the most frequently described in the literature, in 10-50% of cases they may not be found[10].
Diagnosis is made by identifying ovoid yeasts of 2-4 microns in their greatest dimension, in tissue or body fluids, which can delay the start of treatment. The culture, although sensitive and specific, requires several weeks for identification (4 weeks)[10]. Autoantibodies are not reliable[7], but detection of the galactomannan antigen for this fungus is one of the fastest and most sensitive methods, detected in 80-95% of patients with disseminated histoplasmosis or acute pulmonary histoplasmosis. It can be measured in serum, urine, or cerebrospinal fluid. However, this method is not available in all institutions and, at times, its reporting can take long. It has a cross-reaction with blastomycosis, paracoccidioidomycosis, coccidioidomycosis and, rarely, aspergillosis[10]. It is not frequent to document yeast in peripheral blood smears; however, in the case described, it allowed the targeted treatment to be initiated in a timely manner.
In healthy individuals, the infection resolves by itself and does not require treatment, except in those with recent documented exposure, where it is useful to provide early treatment to shorten the duration of the disease and decrease the risk of acute histoplasmosis. In immunosuppressed patients, with moderate to severe acute pulmonary histoplasmosis, or disseminated histoplasmosis, liposomal amphotericin B should be administered (88% response rate vs. 64% with amphotericin B deoxycholate) and continue with itraconazole for 12 months. Cases of disseminated histoplasmosis without treatment are usually fatal, up to 80% mortality. Follow-up should be planned for antigenuria or antigenemia, taking it at the time of diagnosis, at 2 weeks, monthly and every 3-4 months[10].
The current case is an example of opportunistic infection leading to clinical deterioration in a transplanted elderly patient. Some of the risk factors include immunosuppression and age. Timely and adequate start of treatment were fundamental in the patient's response after one year of treatment. She did not have relapses, which occur in 6% of cases[8].
Conclusion
Hemophagocytic syndrome and disseminated histoplasmosis are life-threatening diseases that pose special challenges in the diagnosis of the immunosuppressed patient. Therefore, the rate of suspicion should be high, especially in those patients with fever and pancytopenia. Diagnosis in this population presents difficulties, which may delay the onset of antimycotic coverage. The urine antigen has a sensitivity close to 90%, being a useful and available tool. In the case presented, the peripheral blood smear was determinant for diagnosis. Early initiation of treatment significantly improves clinical outcomes.











 text in
text in 




