What do we know about this problem?
Spinal anesthesia guided by anatomical landmarks has been reported as inaccurate because it does not consider anatomical variants or acquired alterations. Additionally, there is a potential increase in the risk of spinal hematoma, post dural puncture headache and transient or permanent neurological déficit.
As for the use of ultrasound to guide spinal anesthesia, it has been found to make the process easier, requiring less lumbar puncture attempts and improving pain scores and discomfort in elderly patients.
What is new from this study?
The results suggest that US-guided neuraxial anesthesia in the elderly is safe and could facilitate neuraxial block on first attempt.
INTRODUCTION
Spinal, epidural and caudal neuraxial anesthesia provide a combination of sympathetic, sensory or motor block as a function of dose, concentration or volume of the local anesthetic, with a wide range of clinical applications in surgical procedures, acute postoperative pain treatment and chronic pain relief 1.
Traditionally, the spinal anesthesia technique is based on the use of anatomical landmarks for the identification of the interspinous space. These include the posterior midline, iliac crests and the line that connects them (Tuffier's line), generally associated with the vertebral body of L4 or the L4-L5 intervertebral space, and the spinous processes, although with very low concordance 2. The difficulty increases depending on the degree of expertise and the ability to identify anatomical surfaces by palpation, which can be limited in obese or elderly patients 1,3,4. Moreover, multiple puncture attempts may cause patient discomfort and increase the risk of spinal hematoma, post dural puncture headache (PDPH) and neurologic deficit 5-7.
Recently, ultrasonography (US) has been used to identify landmarks before spinal anesthesia 8-10, as it allows to assess spinal anatomy and identify any abnormalities in this axial structure 11,12. It also enables localization of the most adequate intervertebral space for easy access with the spinal needle, and to identify the depth at which it should be inserted 13,14. Moreover, nerve roots and conus medullaris position can be localized in order to determine the safest access level with a higher probability of success 15. The aim is to reduce the number of attempted punctures and associated risks in patients with difficult access for spinal anesthesia 9,16,17.
Despite the safety profile of neuraxial anesthesia, risks and adverse effects still exist. The most common complications include PDPH, dural hematoma formation, lumbar pain and secondary spinal cord injury, as well as low success rate (as low as 30%) with the adequate identification of the puncture level using anatomical landmarks 18. Risk factors for these types of complications have been divided into three groups: patient-related factors such as hemostatic or spinal disorders; procedure-related factors such as complicated spinal anesthesia, including the main risk of retrieving bloody cerebrospinal fluid; and drug-related factors such as the use of anticoagulants 19. According to Lagerkranser et al. 19, the risk of spinal hematoma is higher in patients over 60 years of age and more significantly so in women 19.
The introduction of US in neuraxial anesthesia has improved patient and clinical safety because of accurate identification of the adequate puncture site, the midline and the intervertebral space, with a higher rate of success on first attempt and a lower number of needle passes 18 - the latter having been described as a risk factor por PDPH, spinal hematoma and lumbar pain 18,20. The aim of US guidance during spinal anesthesia (SA) is to visualize and measure the puncture site, thus avoiding multiple attempts, particularly in patients with a difficult anatomy 14,18. It is worth mentioning that, although the use of US could add time to the spinal anesthesia procedure, patients benefit from increased comfort and a higher probability of a successful puncture which, according to the literature, results in lower rates of associated lumbar pain 13,18.
US in spinal anesthesia is extremely useful, especially in older patients in whom age-related technical difficulties can be expected because of degenerative changes, interspinal ligament calcification or acquired spine deformities. Acquiring ultrasound images before spinal anesthesia through the median and paramedian approaches in older patients undergoing orthopedic surgery reduces the number of punctures, making the procedure easier and improving pain scores and patient discomfort 21,22. Against this background, the purpose of this study was to determine which spinal anesthesia technique (US vs. anatomical landmark guidance) results in higher success rates in the elderly. The secondary objectives were to identify and document complications arising during US or anatomical landmark-guided anesthesia, as well as the number of puncture attempts needed to achieve successful spinal anesthesia.
METHODS
Study design and participants
Prospective observational study of patients over 65 years of age scheduled for elective or urgent surgery under spinal anesthesia admitted between October 2021 and November 2022 to Hospital Universitario del Valle, a level III institution, and Fundación Hospital San José Buga. Convenience sampling was used. The study was approved by the ethics committee of Hospital Universitario del Valle with record number 011-2021. The inclusion criteria were patients undergoing orthopedic surgery, umbilical and inguinal hernia repair, open or transuretral prostatectomy, pelvic floor surgery, older than 65 years of age and American Society of Anesthesia classification I - III, with optimal cognitive ability to collaborate with positioning and who had signed the informed consent to undergo the technique and participate in the study.
Exclusion criteria were with at least one of the following: patients with contraindication for subarachnoid anesthesia, a history of lumbar surgery, a history of allergic reaction to local anesthetics, or refusal to participate.
Patients were assigned in alternating order to one of the two groups as they came (one group with subarachnoid anesthesia guided by anatomical landmarks and a second group with US guidance). The median and paramedian approaches were used, depending on the choice of the anesthetist performing the procedure. The choice of guidance was also left to the anesthetist. This article is reported in accordance with the STROBE tool.
Standard procedure
In all patients, the procedure was performed after obtaining the informed consent, and establishing peripheral venous access and basic monitoring as recommended by ASA, using pulse oximetry, electrocardiography and non-invasive blood pressure monitoring. Moreover, all usual asepsis and antisepsis measures were implemented, and the ultrasound probe was wrapped in sterile material. The procedure was performed by two anesthetists experienced in both US and anatomical landmark-guided anesthesia who were not involved in the study. Their experience having performed at least 50 US-guided procedures was confirmed. For both groups, the approach was based on mapping, assessing the best puncture space and defining which space was being measured.
In the US-guided group the steps included equipment preparation (Sonosite M Turbo machine), patient positioning, identification of the spinous processes and placement of the convex ultrasound probe (2-5 mHz) initially on the sacrum on a longitudinal plane at the level of the laminae, identification of the intervertebral space, and selection of the puncture site based on the widest exposed space. A transverse view was acquired of the intervertebral space, taking into account the best probe angulation to visualize the space and measure the distance between the skin and the subarachnoid space (Figure 1). Finally, spaces and puncture site were marked and the puncture made with a Quincke # 26 or 27 needle (Figure 2).
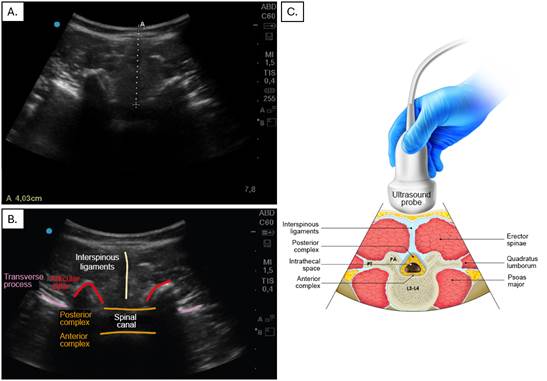
A. Transverse view of the lumbar spine, with the dotted line representing the distance between the skin and the subarachnoid space. B and C. Transverse view showing the various structures along this plane: interspinous ligaments, transverse process, posterior and anterior complex, and spinal canal. Source: Authors.
Figure 1 US-guided anesthesia.
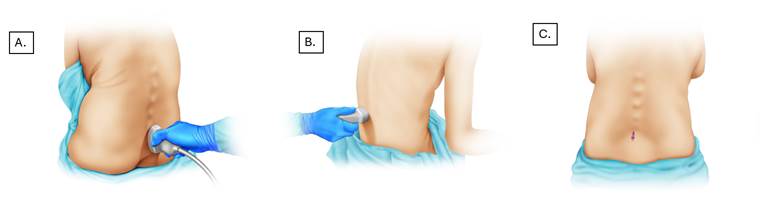
A. The probe is initially placed at the level of the sacrum with a caudal-cranial orientation for better space localization. B. Identification and measurement of structures in the transverse plane. C. Lumbar puncture taking into account distance and scanning angle. Source: Authors.
Figure 2 US-guided anesthesia.
In the group with anatomical landmark guidance, the US equipment was prepared with the low frequency transducer after positioning the patient in sitting or lateral decubitus position - according to the preference of the treating anesthetist. The midline was then identified by means of palpation of the spinous processes and intervertebral spaces; the L4-L5 space location was estimated by drawing an imaginary line between the iliac crests (Tuffier's line) 2. Once the right space was localized, a Quincke 26 or 27 gauge needle was used to perform the puncture (Figure 3). Local anesthetic was administered and the puncture site was confirmed with ultrasound, outlining the intervertebral spaces from the sacrum up to the puncture site.
Data collection
The variables of interest measured were collected by the anesthetist in charge of performing the puncture and by the licensed practical nurse who were aware of the study and its purpose, and using a stop watch available in the operating room. The following variables were taken into consideration: lumbar puncture site, US confirmation of the site, number of punctures, number of redirections, time in minutes needed to establish anesthesia - from the moment anatomical landmarks were ascertained to the intrathecal administration of the local anesthetic - and puncture landmarks.
Statistical analysis
A descriptive analysis of the data was performed, making a distinction according to the anesthesia technique used (US group and anatomical landmark group) and number of puncture attempts (one or more than one). Quantitative variables were presented as mean and standard deviation, or median and interquartile range (IQR), depending on whether the normality assumption was met or not. Qualitative variables were presented in frequency and proportion tables. The chi-square test or the Fisher's exact test was used when both variables were categorical, and the Mann-Whitney U test or the Student t test was used for comparing categorical and quantitative variables.
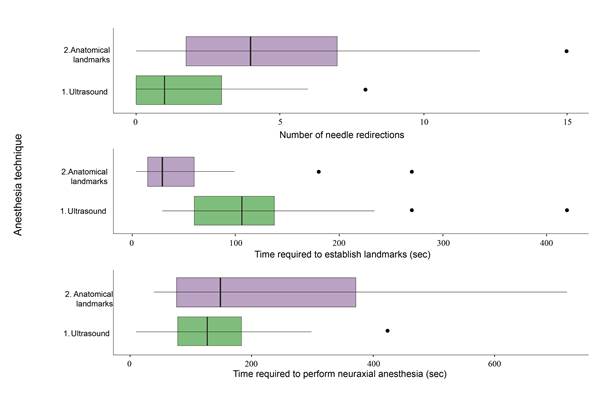
The time required to perform the anesthesia procedure as well as the number of needle redirections were also assessed. Since outcome variables were quantitative, central trend measurements were used, considering a statistical significance level equal to 0.05 or lower. The variables that were considered relevant in the descriptive analysis were later used to build box and whisker plots.
Finally, relative risk (RR) for sex, age, anesthesia technique, body mass index (BMI) and position at the time of performing the anesthesia was estimated, with success being defined as puncture on first attempt. Additionally, a binary response model was adjusted in order to estimate RR.
RESULTS
The study included 80 patients with a mean age of 78.5019.04 (50 women and 30 men), with associated comorbidities such as diabetes mellitus (25%), hypertension (58%), and chronic kidney disease (11%) (Complementary material 1). In terms of puncture numbers, success in achieving spinal anesthesia on first attempt was higher in the US group (US 72.5% vs. anatomical landmark group 45.0%; p=0.02). The number of needle redirections was also lower in the US group (US group median = 1; landmark group = 4); the time taken to localize the puncture site was longer in the US group, but the total mean time to achieve anesthesia, measured from the time of landmark establishment to the administration of the drug, was 137 (78.25243.75) with no difference between the two groups (Figure 4).
Patients were positioned either on lateral decubitus or in sitting position depending on the preference of the treating anesthetist, with the sitting position being used more frequently in the US group (75% vs. 42% p=0.01) (Table 1).
Table 1 Variable analysis by groups.
IQR: Interquartile range; Sec: Seconds; US: Ultrasound.
Source: Authors.
No complications such as spinal hematoma, lumbar pain or PDPH were observed or reported in either group. Additionally, RR was estimated by sex, age, anesthetic technique, BMI and position at the time of puncture. A level of statistical significance was found for success on first attempt with US and for the sitting position (Table 2). A binary response model adjustment was used to estimate adjusted RR.
Table 2 Risk estimation at the time of puncture considering success as puncture on first attempt.
| OR estimations | |||
|---|---|---|---|
| Variable | Crude RR (95% CI) | Adjusted RR (95% CI) | p value |
| Age | 1.1(0.76-1.61) | 1.02 (0.97-1.08) | 0.47 |
| Sex | 0.97(0.66-1.41) | 1.98(0.66-6.45) | 0.24 |
| Anesthesia technique: US | 0.62(0.42-0.92) | 0.35(0.11-0.99) | 0.05 |
| Body mass index | 0.93(0.64-1.34) | 1(0.88-1.13) | 0.96 |
| Position: Sitting | 0.54(0.34-0.86) | 0.28(0.09-0.79) | 0.02 |
Source: Authors.
In the anatomical landmark group, puncture site confirmation was made after spinal anesthesia placement, and inconsistency with the previously estimated site was found in 37.5% of patients. This creates the risk of not being able to achieve anesthetic block in the adequate site.
DISCUSSION
The results of this study showed that the use of ultrasound for neuraxial anesthesia in elderly patients is extremely helpful and could be associated with a higher rate of success of spinal anesthesia on first attempt.
The geriatric population is growing worldwide and these are patients who usually present with multiple medical comorbidities and underlying bone fragility because of loss of bone mineral density and muscle mass, creating a higher risk for perioperative and postoperative complications 23. Patient age has been found to be an independent predictor of increased technical difficulty with neuraxial anesthesia as a result of degenerative changes affecting the neural axis 24. Narrowing of interspinous and intervertebral spaces can be found in elderly patients due to interspinous ligament ossification and facet joint hypertrophy 25,26. This difficulty identifying the interspinous space is compounded by the difficulty in achieving adequate lumbar flexion, limiting adequate patient position for the administration of spinal anesthesia 27-29.
Although additional time is usually required for finding anatomical landmarks with the use of US, when total time for spinal anesthesia is analyzed, it appears that there are no differences when compared with the use of anatomical landmarks to guide the technique. This is probably due to the higher number of punctures and needle redirections needed with the latter.
The use of ultrasound to approach the neural axis has been described both in the context of acquiring images before the spinal anesthesia procedure and site marking, as well as in real-time needle guidance, with the majority of studies conducted in the obstetric population. However, we present results in elderly patients that support the use of US as a safe and effective alternative to guide spinal anesthesia. It is important to highlight that, unlike the anatomical landmarks group, the sitting position was the predominant position in the US group.
This observational study has limitations, including the absence of standardized surgical indications or associated comorbidities which could make positioning more challenging. Sample size was small and, because of its design, the study was not randomized or blinded. Data were collected in two hospitals, limiting generalization of the findings; moreover, potential selection bias could have been induced because anesthetists could have chosen US in those patients with additional predictors of difficult lumbar approach besides age. There were no major complications associated with the procedure. A cost-effectiveness analysis comparing the US-guided technique vs. the technique based on anatomical landmarks was not performed; however, ultrasound machines were available in both institutions, so no procurement costs were involved. Randomized controlled studies with larger sample sizes are required in order to confirm the superiority of ultrasound guidance when compared with the use of anatomical landmarks to guide spinal anesthesia.
CONCLUSION
The use of ultrasound guidance for procedures such as spinal anesthesia in elderly patients is an option that could improve success on first puncture, with no significant impact on the time required to achieve final anesthesia. However, due to the limitations of this study, it is important to conduct additional, more robust research with larger sample sizes like a randomized study, in order to validate these results.
ETHICAL RESPONSIBILITIES
Ethics committee approval
This study was approved by the ethics committee of Hospital Universitario del Valle, as set forth in Minutes 011-2021 of the 21st of March, 2021, in Cali, Colombia.
Protection of human and animal subjects
The authors declare that no experiments were performed on humans or animals for this study.











 text in
text in